Abstract
INTRODUCTION
The incidence of coronary artery anomalies (CAAs) varies from 0.2% to 8.4%. Knowledge of such anatomical variations is important as coronary procedures are regularly performed these days. We aimed to find the coronary dominance pattern, intermediate artery (IMA) frequency and CAA incidence in our clinic, and compare them to those in the literature.
METHODS
The medical reports of 5,548 patients who had undergone coronary angiography (CAG) between 2005 and 2009 were retrospectively investigated. Dominance pattern and presence of IMA and CAA were recorded. CAAs were described using two different classifications: Angelini and Khatami’s classification, and a new modified classification that was derived from Angelini and Khatami’s classification. Some procedural details and clinical features of the patients with CAA were also investigated.
RESULTS
Coronary dominance pattern was: 81.6% right coronary artery, 12.2% circumflex artery and 6.2% co-dominant. IMA was present in 613 (11.0%) patients. The incidences of overall anomaly were 2.7% and 1.4%, according to the different classifications. Absent left main coronary artery, which was the most common anomaly in the present study, was found in 51 (0.9%) patients. Incidences of myocardial bridge, coronary arteriovenous fistulae and aneurysms were 1.1%, 0.2% and 0.3%, respectively.
CONCLUSION
CAAs are generally asymptomatic, isolated lesions. Some may lead to anginal symptoms, myocardial infarction or sudden death. We found that CAA was associated with increased radiation and contrast exposure in patients who underwent CAG. This risk could be reduced if appropriate catheters were designed and training programmes on ostial cannulation were developed.
INTRODUCTION
With an increasing number of coronary angiography (CAG) procedures, coronary invasive procedures and cardiac bypass surgeries performed each day, knowledge of the variations, anomalies and anatomical pattern of coronary arteries is gaining in importance. Although many individuals have a normal coronary anatomy, variations and anomalies are not unusual, and may lead to complications during procedures.(1)
There are two main coronary arteries that supply oxygenated blood to the myocardium – the left main coronary artery (LMCA) and the right coronary artery (RCA). The LMCA originates from the left sinus of Valsalva (SV), while the RCA originates from the right SV. There is typically no artery arising from the posterior SV. The LMCA bifurcates into the left anterior descending (LAD) artery and the circumflex artery (CXA). An additional artery called the intermediate artery (IMA) may arise at the bifurcation of the LMCA, forming a trifurcation.
The LAD artery runs in the anterior interventricular sulcus, providing the penetrating septal branches. The left CXA runs along the left atrioventricular sulcus and gives rise to at least one obtuse marginal (OM) branch, while the RCA lies in the right atrioventricular sulcus and gives rise to the acute marginal branch. The IMA, which supplies the left ventricular free wall, is located anterior to the first OM artery and posterior to the first diagonal artery. It can also originate from the proximal part of the LMCA, LAD artery or CXA. In some cases, it is not possible to distinguish between the IMA and OM artery using anatomical or angiographic examinations. However, an important point of distinction is that the left ventricular free wall is supplied by the IMA.(1-3)
The artery that supplies the posterior descending artery determines coronary dominance. Approximately 70%–80% of the general population is right-dominant (i.e. supplied by the RCA), while 5%–10% is left-dominant (i.e. supplied by the CXA) and 10%–20% is co-dominant (i.e. supplied by both the RCA and CXA).(3-5) A more accurate definition of dominance refers to the arterial supply to the atrioventricular nodal artery, which is generally supplied by the RCA.(3-5)
Typically, there are two coronary ostia. However, in some cases where the LMCA is absent, three ostia can be detected. In individuals with such a condition, the LAD artery and CXA originate from different ostia. Absence of the LMCA is a common anomaly that can be detected in 0.4%–8% of the population.(4,5)
Coronary arteries can be anatomically categorised into three groups based on their anatomical features: normal coronary anatomy, anatomic variations of the coronary artery and coronary artery anomalies (CAAs). CAAs, which are congenital disorders in the coronary anatomy that are observed in less than 1% of the general population, are evaluated using CAG series. Angelini identified a set of criteria that allowed the systematic assessment of coronary arteries and hence the description of normal coronary anatomy.(6) Angelini introduced the concept of normal variant versus anomaly based on a statistical definition of what constitutes the normal range (coronary pattern observed in 99% of an unselected population) and CAAs (coronary patterns observed in less than 1% of the study population).(6) This systematic anatomic approach is probably the most valid classification method for CAA.
CAAs are generally isolated lesions and are rarely related to congenital heart diseases such as mitral valve prolapse, bicuspid aortic valve and aortic coarctation. They are also not associated with an increased risk of coronary atherosclerosis. Although they are present at birth, relatively few CAAs are symptomatic during childhood. Most remain asymptomatic throughout life and may be encountered as incidental findings during CAG or autopsies. While CAAs have not been strongly associated with congenital heart diseases and coronary atherosclerosis, recognition of CAAs is important for the appropriate diagnosis and management of patients undergoing coronary angioplasty or cardiac surgery.(7,8) Some CAAs are associated with anginal syndrome, myocardial infarction and sudden death, and therefore have to be treated.(9-11)
The incidence of CAAs is generally reported to be about 0.2%–1.3%.(7,12-17) However, in a prospective angiographic study that was performed using a strict predefined criteria and classification scheme, the incidence was found to be 5.6% in 1,950 consecutive cases.(18) As CAG is an easily accessible and commonly used technique worldwide, the probability of identifying CAAs may increase. The coronary dominance pattern, presence of IMA and incidence of CAAs may vary depending on the population studied. In the present study, we aimed to identify the coronary dominance pattern, IMA frequency and incidence of CAAs among the patients in our clinic who had undergone CAG, and compared our results with data found in the literature.
METHODS
The CAG reports of 5,548 patients (3,858 men, 1,690 women) who underwent CAG in our clinic between 2005 and 2009 were retrospectively investigated. Their catheterisation reports were also analysed. Patients who were identified to have CAAs were selected for further assessment. The CAG images were reviewed by four independent investigators and re-evaluated if necessary. Patients who had CAAs that occurred as part of complex congenital heart diseases were excluded from the present study.
Coronary dominance pattern and the presence of IMA and CAA were recorded. CAAs were grouped according to the systematic anatomic approach developed by Angelini and Khatami, which is recognised as the most valid classification for CAA.(19) Based on this approach, CAAs were classified into the following seven groups:(4,6,19) (a) anomalous pulmonary origins of the coronary arteries; (b) anomalous aortic origins of the coronary arteries; (c) congenital atresia of coronary arteries; (d) myocardial bridging; (e) coronary arteriovenous fistulae (CAFs); (f) coronary artery aneurysms; and (g) coronary stenosis. A simplified modification of Angelini and Khatami’s classification is currently used in clinical practice.(19) It divides CAAs into two groups: Group A, anomalies of origin and distribution; and Group B, intercoronary communications and coronary artery fistulae. Both classification systems were used to evaluate the CAAs in the present study.
A myocardial bridge (MB) is a condition in which a coronary artery tunnels through the myocardium rather than resting on top of it. A CAF is an abnormal connection between a coronary artery and another structure, most commonly a venous structure or a chamber on the right side of the heart. Abnormal dilatation of a part of the coronary artery is called coronary artery ectasia or coronary aneurysm. Coronary artery ectasia is the dilatation of a coronary artery segment to a diameter 1.5 to two times that of the adjacent segment, whereas coronary aneurysm is the dilatation of a coronary artery segment to a diameter more than two times that of the adjacent segment.
Incidence proportion (i.e. cumulative incidence) is the number of new cases within a specified time period divided by the size of the population initially at risk. In the present study, the angiographic incidence of CAAs was calculated as the number of CAAs divided by the total number of patients enrolled in the study (n = 5,548). Anomaly incidence is defined as the percentage of a specific anomaly among the total CAAs detected in the study.
Patients who had CAAs, according to the modified classification system for CAA, were re-analysed in the present study. Unlike other studies found in the literature, some procedural details (including fluoroscopy time, the volume of contrast media used and the number of catheters used) and clinical features of patients with CAA were also investigated.
RESULTS
The mean age of the 5,548 patients who had undergone diagnostic CAG between 2005 and 2009 was 61.3 ± 11.3 years. Most of the patients were men (69.5%, n = 3,858). The most frequent CAG indications were chest pain, positive stress test and wall motion abnormality on echocardiography. The coronary dominance pattern in our study was 81.6% RCA, 12.2% CXA and 6.2% co-dominant. There was no significant difference in the coronary dominance pattern between the genders. IMA was detected in 613 (11.0%) patients and found to be significantly more frequent in male patients than female patients (13.0% vs. 6.7%, p < 0.001).
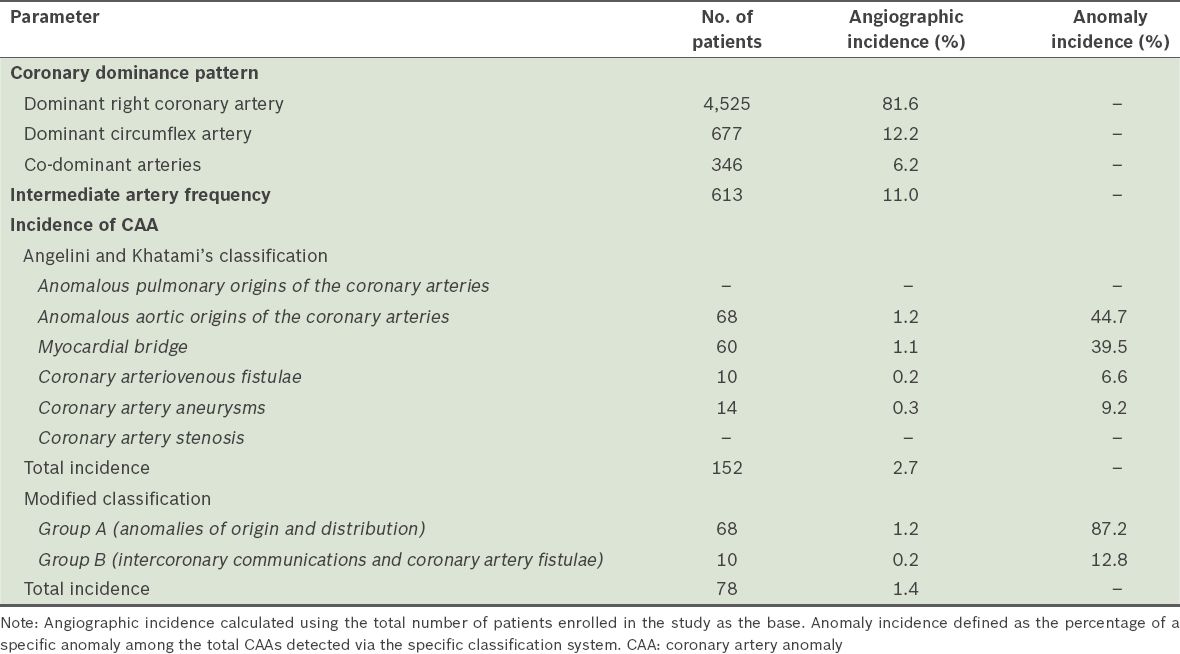
According to Angelini and Khatami’s classification, the overall incidence of CAAs was found to be 2.7%. According to the modified classification, the incidence of Group A and Group B anomalies was 1.2% and 0.2%, respectively. Based on the modified classification, 68 (87.2%) patients had anomalies of origin and distribution (i.e. Group A), while 10 (12.8%) had intercoronary communications and coronary artery fistulae (i.e. Group B). We found the LMCA origin and distribution anomaly in 52 patients, making it the most common anomaly observed in the present study. As the modified classification does not accept MB and coronary artery aneurysms as coronary anomalies, the incidence of CAAs was found to be 1.4%. The coronary dominance pattern, IMA frequency and incidence of CAAs are summarised in
Table I
Coronary dominance pattern, intermediate artery frequency and incidence of CAAs in the study cohort (n = 5,548), according to the two classification systems.
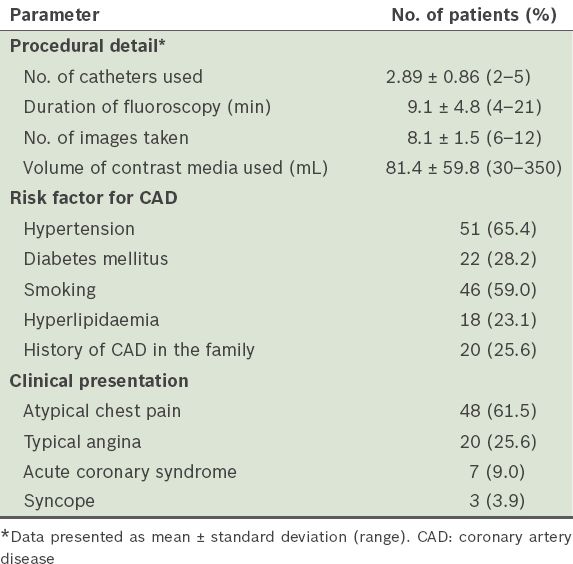
The LMCA was the most common anomalous vessel in the present study. The incidence of LMCA absence was 0.9%. In ten patients, the LMCA originated from the CXA, while in two patients it originated from the LAD artery; only one patient had an LMCA that originated from the right coronary circulation (i.e. the right SV or RCA). The RCA originated from the left coronary circulation (i.e the left SV or left coronary arteries) in four patients. CAF was detected in 10 (0.2%) patients – six originated from the RCA and four from the CXA or LAD artery. MB was detected in 60 (1.1%) patients and at a higher frequency among the male patients than the female patients (1.4% vs. 0.4%, p = 0.02). Coronary ectasia was observed in 352 (6.3%) patients; a higher frequency was again observed among the male patients than among the female patients (7.8% vs. 3.0%, p < 0.001). Aneurysms were detected in 14 (0.3%) patients and slow coronary flow was diagnosed in 60 (1.1%) patients. Anomalous origin of the coronary arteries from the pulmonary artery or atretic coronary arteries was not observed in the present study. In our analysis of the 78 patients with CAA (based on the modified classification), the mean age of the patients was 57.0 ± 10.4 (range 34–79) years and 48 (61.5%) of them were men. The most common clinical presentations of the patients with CAA were: atypical chest pain (61.5%); typical angina (25.6%); acute coronary syndrome (9.0%); and syncope (3.9%). Selected procedural details, as well as the demographic characteristics and clinical features of the patients with CAA, are presented in
Table II
Selected procedural details, demographic characteristics and clinical features of the patients with coronary artery anomaly (n = 78).
DISCUSSION
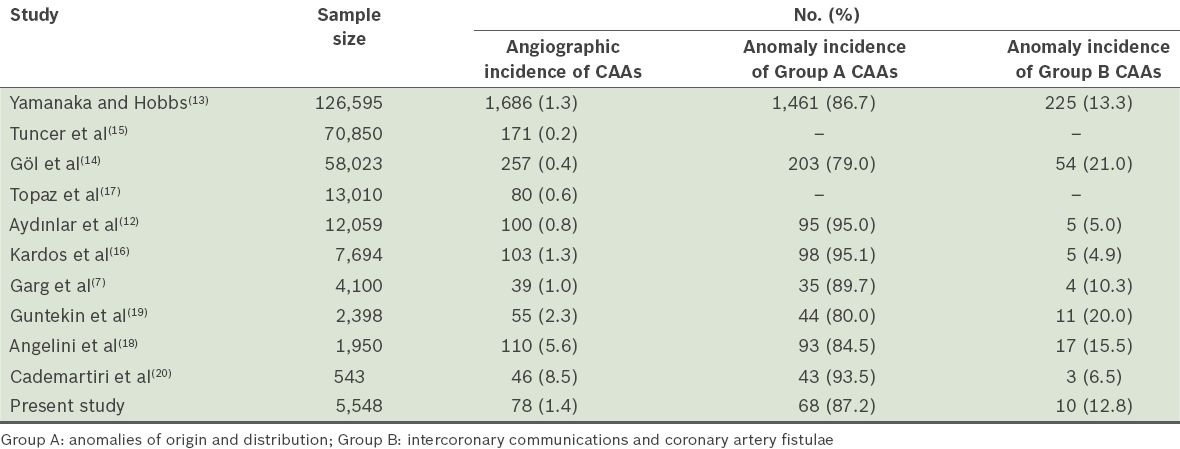
The coronary dominance pattern, presence of IMA and incidence of CAAs may vary in different CAG series, depending on the characteristics of the study population. The coronary dominance pattern in the present study was 81.6% RCA, 12.2% CXA and 6.2% co-dominant, consistent with that found in the literature.(20) The dominance pattern was similar between genders in the present study. IMA was present in 613 (11.0%) patients, a higher percentage than that reported in the literature.(5) IMA frequency was also found to be high in a study that used 64-slice computed tomography (CT) CAG.(20) In the present study, IMA was observed more frequently among the male patients. CAAs were found in 0.2%–1.3% of CAGs and 0.3% of autopsies in the literature.(7,12-17) However, in one particular study that involved the evaluation of 543 patients’ CT CAGs, the incidence of CAAs was found to be 8.4%.(20) This high incidence could be due to the inclusion of ectasia, aneurysms and MBs in the definition of CAAs in that study. The incidences of CAAs reported in several large-scale studies are summarised in
Table III
Incidences of coronary artery anomalies (CAAs) in the literature.
The overall incidence of CAA in our study was 2.7% based on Angelini and Khatami’s classification. As MB and coronary artery aneurysms are not classified as CAAs in the modified classification, the incidence of CAAs was found to be only 1.4% using the modified classification. Based on the modified classification, 87.2% of the CAAs were abnormalities in the origin or distribution of a coronary artery (i.e. Group A) and 12.8% were abnormal fistulae (i.e. Group B). The incidences of CAAs in the present study, according to both classifications, were higher than most of the reported values in the literature.(7,12-17) The incidence of CAF was also relatively high in the present study.(7,12,16,20)
The results of the present study are very similar to those of the largest study on CAAs, which was conducted by Yamanaka and Hobbs.(13) The authors retrospectively reviewed 126,595 CAG reports of patients from the Cleveland Clinic Foundation between 1960 and 1988. A total of 1,686 patients with CAAs were found, giving a 1.3% incidence of CAA.(13) Most (80%) of the CAAs were benign and asymptomatic, with only 20% of them being clinically significant. Yamanaka and Hobbs reported separate origin of the LAD artery and CXA from the left SV, anomalous origin of the CXA from the right SV, ectopic origin of the coronary artery from the posterior SV, anomalous origin of the coronary artery from the ascending aorta, absent circumflex, intercoronary communications and small coronary artery fistulae as benign anomalies.(13) Ectopic coronary origin from the pulmonary artery, ectopic coronary origin from the opposite aortic sinus, single coronary artery and large coronary fistulae were reported as clinically significant, as these anomalies may be associated with anginal syndromes, myocardial infarction, arrhythmias and sudden death.
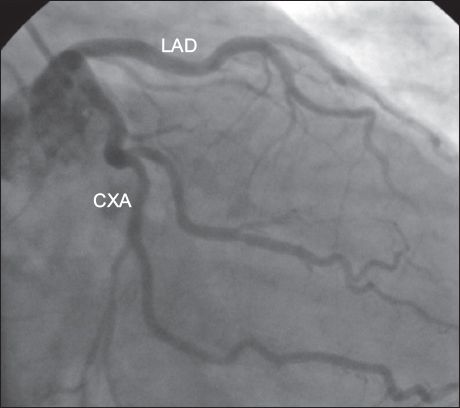
Similar to some large studies in the literature,(12,13,16,17) separate origin of the LAD artery and CXA from the left SV (i.e. absence of the LMCA) was the most common anomaly in the present study. Angiographic incidence of the absence of the LMCA was reported to be 0.23% and 0.58% in two studies performed in Turkey.(12,19) In the present study, the LMCA was the most common anomalous vessel and the incidence of absence of the LMCA (
Fig. 1
Angiogram shows the separate origin of the left anterior descending (LAD) artery and circumflex artery (CXA) from the left sinus of Valsalva, with absence of the left main coronary artery.
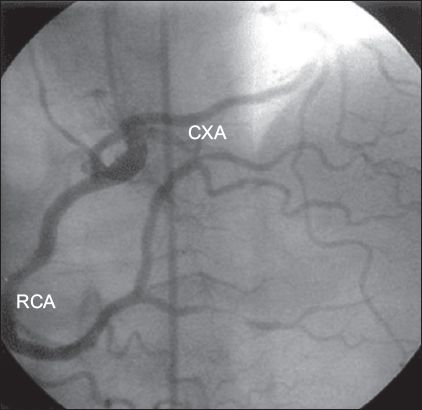
Coronary arteries may arise from the contralateral coronary sinus or as an early branch of other coronary arteries. Anomalous origin of the CXA from the right coronary circulation (i.e. the right SV or RCA), which is the most frequent anomaly, is generally a benign condition.(14,15) In two large studies, the CXA was found to be the most common anomalous vessel (34%–51.1%).(14,15) In the present study, ten patients had anomalous origin of the CXA from the right coronary circulation (
Fig. 2
Angiogram shows the circumflex artery (CXA) originating from the right coronary artery (RCA).
Anomalous origin of the RCA from the left coronary circulation (i.e. the left SV or left coronary arteries) is also a common coronary anomaly. It was observed to be the most frequent anomaly (49%) in one retrospective trial.(7) In Yamanaka and Hobbs’ trial, the angiographic and anomaly incidences of this anomaly were 0.1% and 10%, respectively.(13) Studies from Turkey reported the incidence of this anomaly to be between 3.4% and 22% among CAAs.(12,19) In the present study, four patients had anomalous origin of the RCA from the left coronary circulation. The incidence of this anomaly was 5.1%, which is consistent with the literature. Physicians should be aware of this anomaly, as it is one that may lead to sudden death.(21)
Anomalies of the LMCA or LAD artery have more clinical significance than those of the CXA. In the present study, two patients had anomalous origin of the LAD artery from the right coronary circulation. Although the incidence of origin and distribution anomalies of the LAD artery was generally reported to be quite low in the literature, Tuncer et al found the angiographic incidence of this anomaly to be 0.017%.(15) As LMCA originating from the right SV has been reported to result in sudden death and myocardial ischaemia, patients found to have this anomaly should be followed closely even if they are asymptomatic. Only one patient had this anomaly in the present study. Single CAA, which is a clinically important anomaly because it can lead to sudden death, was not detected in our patients.
A coronary artery, such as the LMCA (most common) and the LAD artery or RCA (less common), may arise from the pulmonary artery. This is a fatal condition and almost 90% of such patients die during infancy. In patients who survive, retrograde filling of the anomalous artery occurs through collaterals, draining into the pulmonary artery (via a left-to-right shunt). This condition may cause coronary steal and consequently angina, infarction and heart failure. The anomaly requires surgical repair.(13) In the present study, this anomaly was not observed.
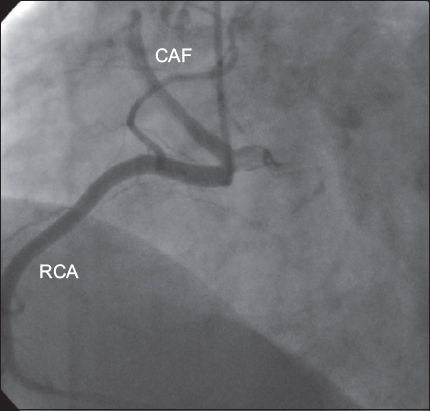
A CAF is an abnormal connection between one of the coronary arteries and another structure. The frequency of this condition is 1/50,000 at birth and 1/500 at cardiac catheterisation.(14,19) More than 50% of these fistulae have a connection with the RCA. Most of them drain into the right ventricle (40%), right atrium (25%) or coronary sinus, while some drain into the pulmonary artery, left atrium or left ventricle.(12,13) In general, the shunt is small and the patients are asymptomatic. However, if the shunt is large, pulmonary hypertension, congestive heart failure, bacterial endocarditis, rupture and myocardial ischaemia may occur.(13) In some series, fistulae were as common as CAAs (21%).(14) In the present study, we observed ten CAFs and the angiographic incidence of CAFs was 0.2%. Six had a connection with the RCA (
Fig. 3
Angiogram shows a coronary arteriovenous fistula (CAF) originating from the right coronary artery (RCA).
Based on autopsy findings, the incidence of MB is estimated to be 1%–5% in the general population.(19,20) It is important to know the extent of the obstruction during systole when evaluating the severity of this condition. If there is less than 50% blockage, the condition is probably benign. However, if the obstruction causes at least 70% blockage, anginal symptoms can result.(22-24) As more than two-thirds of blood flow in the coronary system occurs in diastole, the MB, which causes systolic narrowing of a coronary artery, is not expected to lead to impairment in the myocardial perfusion, ischaemia or angina. Although the pathophysiology of ischaemia and angina in MB is not well known, ischaemia in MB is considered to be associated with the severity of systolic compression, diastolic filling time and heart rate. For example, tachycardia could provoke an ischaemic effect by shortening the diastolic phase and increasing the importance of systolic blood flow. There are three main considerations that are associated with ischaemia in MB:(24-26) (a) the ‘spill over’ phenomenon, which is characterised by the persistence of narrowing in early diastole. The more severe the systolic narrowing, the higher the likelihood of ‘spill overs’ occurring; (b) periods of tachycardia, in which there is more systolic compression and systole occupies a greater percentage of the cardiac cycle due to shortening of the diastolic filling period; and (c) microvascular compression. Clinically important MB can cause complications such as vasospasm, angina pectoris and arrhythmias. The classification of MB as a CAA is controversial. While MB was not included in the definition of CAAs in some large studies,(12,14) it was regarded as a CAA in two studies conducted in Turkey.(15,19) In the present study, 60 (1.1%) patients had MB.
Coronary artery ectasia and aneurysm are described as abnormal dilatations of a part of the coronary artery. They are rare anomalies whose aetiologies are unclear, but they are usually considered to be variants of coronary atherosclerosis.(1,12,17) They may be associated with inflammatory diseases, connective tissue diseases and some congenital diseases.(27,28) In the present study, 352 (6.3%) of our patients had ectasia and 14 (0.3%) had aneurysms. Both of these conditions showed a male dominance in the present study, similar to that observed in coronary artery diseases. As both conditions have similar clinical prognosis, they have to be treated as coronary artery disease.(1,12,17)
The incidence of CAAs varies, ranging from 0.2% to 8.4%.(7,12-20) This may be due to the lack of consensus on whether MBs, ectasia and aneurysms should be classified as CAAs. While MBs and aneurysms are considered CAAs in Angelini and Khatami’s classification, they are not considered CAAs in the modified classification. In the present study, we used both classifications to evaluate the incidence of CAAs. Although CAAs are rare causes of sudden death, anomaly of a single coronary artery, an LMCA originating from the pulmonary artery and congenital hypoplastic, stenotic or atretic LMCA may lead to myocardial infarction and sudden death. LMCAs originating from the right SV and RCAs originating from the left SV are also associated with sudden cardiac death.(12,21)
The relationship between the anomaly and the presenting symptoms is often vague. It is important to fully understand the prognosis of CAAs in order to apply the appropriate treatment. As the clinical relevance of different anomalies may vary greatly, cost efficiency is a major concern. Although most CAAs can be detected precisely using CAG, the cost incurred and the invasive nature of this procedure limits its use for screening. In addition, fewer than 10% of the patients who experience sudden death due to CAA have symptoms related to the anomaly, with 60%–90% of these patients showing no clinical manifestations.(4)
The relationship between the volume of contrast media used and risk of the patient developing contrast-induced nephropathy is controversial. Some studies reported no relationship between these two factors, whereas others suggest that volume of contrast media used is an independent predictor for the occurrence of contrast-induced nephropathy.(24,29,30) However, there is a consensus that low doses of contrast media should be used and repetitive injections should be avoided. The definition of ‘low dose’ varies in different studies, but it is generally defined to be < 70–100 mL.(29,30) The results of the present study indicate that CAAs are associated with increased radiation and contrast exposure in patients who underwent CAG, owing to difficulties in ostial cannulation. As CAAs are rare, the experience of clinicians is also an important factor that influences the number of catheters used, the duration of fluoroscopy and the volume of contrast media used during the procedure. To reduce these disadvantages in the management of CAAs, it could be beneficial to develop appropriate catheters for CAAs, as well as implement training programmes on ostial cannulation for clinicians.
In conclusion, the coronary dominance pattern, presence of IMA and incidence of CAAs may vary in different CAG series depending on the characteristics of the study population. The incidence of CAAs ranges from 0.2% to 1.3% in CAG series and has been reported to be 0.3% in autopsy series. In the present study, there was a higher incidence of CAAs in our study population. Although CAAs are generally asymptomatic, isolated lesions that are usually not associated with congenital heart disease or atherosclerosis, some may lead to anginal symptoms, myocardial infarction and sudden death. The recognition of coronary anatomical patterns, variations and CAAs is very important, especially when dealing with patients who are scheduled for coronary angioplasty or cardiac surgery. Further prospective studies are required to evaluate the incidence and prognosis of CAAs.


