Abstract
The spleen is considered ‘the forgotten organ’ among radiologists and clinicians, although it is well visualised on abdominal computed tomography and magnetic resonance imaging. Moreover, the spleen is commonly involved in a wide range of pathologic disorders. These include congenital anomalies, infectious and inflammatory diseases, vascular disorders, benign and malignant tumours, and systemic disorders. In this review, we focus on the key imaging findings of the normal spleen, its variants, as well as relevant congenital and acquired abnormalities. It is of utmost importance to recognise and correctly interpret the variable spectrum of abnormalities that may involve the spleen, in order to avoid unnecessary invasive procedures and to guide adequate treatment.
INTRODUCTION
Compared to imaging evaluation of other upper abdominal organs, requests for imaging of the spleen are relatively rare in daily clinical practice. However, the spleen may be involved in a large variety of congenital and acquired disorders. Significant overlap in imaging features among these various conditions is seen; therefore, clinical information is important. The purpose of this paper is to give a comprehensive overview of splenic imaging.
NORMAL SPLEEN
Microscopic anatomy
The splenic parenchyma consists of lymphatic follicles and reticuloendothelial cells, surrounding the arteries (‘white pulp’) and an interspersed network of vascular sinusoids (‘red pulp’).(1) The ratio of white to red pulp increases with age due to accumulated antigenic exposure and stimulation. There are two circulatory routes through the splenic pulp: open and closed circulation. The closed ‘fast flow’ circulation drains blood directly into the venous sinusoids, flowing together to become trabecular veins.(2) In the open ‘slow flow’ circulation, blood flows into a reticular fibrous framework of the red pulp and/or via the marginal zone of the lymphatic tissue before reaching the sinusoids.(3) These variable circulatory routes through the splenic pulp may explain the unique inhomogeneous early enhancement pattern of the spleen on computed tomography (CT) and magnetic resonance (MR) imaging.(4)
IMAGING MODALITIES
Ultrasonography
Ultrasonography (US) is frequently the first imaging modality used to evaluate the spleen (
Fig. 1
Normal spleen on US. (a) Coronal and (b) axial views of the left upper quadrant show a normal spleen. The black line represents the splenic width, the white line in (a) represents the splenic length and the white line in (b) indicates the splenic depth.

Computed tomography
On unenhanced CT, the spleen is homogeneous with attenuation values ranging between 40 and 60 Hounsfield units (HU). Unenhanced images are mainly used for the detection of splenic calcifications. After intravenous contrast injection, the normal spleen enhances in a mottled pattern during the arterial and early portal venous phases (
Fig. 2
Normal enhancement pattern of the spleen on CT. Axial CT images (a) before and (b & c) after intravenous administration of iodinated contrast material show heterogeneous enhancement of the splenic parenchyma during the arterial phase (as seen in

Magnetic resonance imaging
On T1-weighted images (WI), the normal spleen has a homogeneous low signal intensity that is slightly less than that of the liver and muscle. On T2-WI, signal intensity is higher than that of the liver parenchyma. Splenic signal intensity, however, varies with patient age.(1) In the newborn, white pulp is not yet matured, resulting in a more hypointense signal intensity than the normal liver parenchyma on T2-WI and more isointense signal on T1-WI. Within the first months of life, the imaging characteristics evolve to the normal adult pattern. Pulse sequences used for MR imaging of the spleen are similar to those used for routine liver imaging (
Table I
Imaging sequences used for evaluation of the spleen.

Fig. 3
Normal enhancement pattern of the spleen on MR imaging. (a) Fat-suppressed T1-W image shows the spleen (asterisk) as being isointense to slightly hypointense to the muscle. (b) During the arterial phase after intravenous gadolinium contrast enhancement, the spleen shows a serpentine-cordlike pattern. (c) In the portal phase, there is uniform enhancement throughout the spleen.

SIGNIFICANT SPLENIC VARIATIONS
Splenic clefts, notches and lobules
Splenic lobules are remnants of fetal splenic lobulation, persisting in adult life as variations in normal shape. They are most commonly seen along the medial part of the spleen and are sometimes supplied by a branch of the splenic artery.(7) Splenic notches or clefts in the splenic borders are common and represent remnants of the grooves that separated the fetal lobules (
Fig. 4
Splenic lobulation. Coronal contrast-enhanced CT image shows a splenic lobule (black arrow) and two clefts more laterally (white arrows).

Accessory spleen
Accessory spleens are also known as surnumerary spleens, splenunculi or splenules. They represent normal splenic tissue separated from the main body of the spleen, originating from nonfusion of the splenic “anlage” and being either solitary or multifocal.(8,9) The size varies from a few millimetres to several centimetres. Accessory spleens are seen in 10%–15% of patients, and are usually located near the splenic hilum, the tail of the pancreas, and the gastrosplenic or splenorenal ligaments (
Fig. 5
Accessory spleen adjacent to the pancreatic tail, mimicking a pancreatic mass. (a) Axial contrast-enhanced CT; (b) axial T1-W; and (c) T2-W images show a focal mass (arrows) adjacent to the pancreatic tail, with similar signal intensity and enhancement pattern as the spleen, consistent with an accessory spleen.

Asplenia and polysplenia
Complete absence of the spleen (‘asplenia’) or multiple discrete spleens (‘polysplenia’) are congenital syndromes that are associated with anomalies in viscero-atrial situs. In the management of these syndromes, it is important to search for associated abdominal and thoracic malformations. It should also be noted that in reality, these syndromes are very complex, since they do not have a fixed set of characteristics that are present in all cases.(10) Polysplenia is usually diagnosed in early childhood because of cardiac anomalies. Most patients die by the age of 5 years.(7) The spleens are usually equally sized and may be left- or right-sided, along the greater curvature of the stomach (
Fig. 6
Polysplenia in a 37-year-old woman. Axial contrast-enhanced CT image shows abdominal situs inversus with multiple round splenules (S) in the right upper quadrant, lateral to the stomach. The liver is left-sided and enlarged. The intrahepatic segment of the inferior vena cava (arrow) is on the left of the aorta. Note the slightly enlarged azygous vein (A) and hemiazygous vein (HA).

Splenosis
Splenosis represents ectopic splenic peritoneal implants.(12) In contrast to accessory spleens, splenosis is an acquired condition secondary to seeding and implantation of splenic cells after splenic trauma or splenectomy.(7,13) As with accessory and ectopic spleens, such implants consist of functional splenic tissue, which provides some protection against infection. Usually the implants are multiple, measuring from a few millimetres up to several centimetres in diameter. Occasionally, they occur in extraperitoneal locations, such as the chest. US, CT and MR imaging can be used to detect splenosis. On all these imaging modalities, splenosis shows the same imaging characteristics as a normal spleen. Recognising and reporting splenules is important, as they may mimic mass lesions such as metastatic adenopathy or lymphoma. As with accessory spleens, scintigraphy with Tc-99m sulfur colloid or denatured red cells is the most sensitive technique for detecting ectopic splenic peritoneal implants.(3)
SPLENIC CYSTS
Histopathologically, cystic lesions of the spleen may be classified as primary (or true cysts) and secondary (or false cysts). While true cysts have an epithelial cellular lining, the cyst wall of false cysts is composed of fibrous tissue. Although reliable differentiation between true and false cysts by imaging is usually not possible and is more of academic interest than clinical importance, there are certain imaging features that may suggest a presumptive diagnosis (
Table II
Useful differential diagnostic criteria for imaging characterisation of splenic cysts.

Congenital cysts
Congenital cysts, also called epidermoid or epithelial cysts, comprise approximately 10% of all true splenic cysts worldwide.(3) On US, most cysts are unilocular and homogeneously anechoic, with a thin wall (
Fig. 7
Congenital cyst. (a) US image of a 20-year-old woman with chronic abdominal pain shows a thin-walled anechoic lesion near the splenic hilum (arrow). (b) Axial contrast-enhanced CT image in the same patient shows a round, thin-walled hypodense lesion (arrow).

False cysts
False cysts account for approximately 80% of all splenic cysts.(16) They result from either a previous trauma, infection or infarction.(17) On imaging, false cysts tend to be small in size, well defined, and mostly multilocular. They are usually located close to the splenic capsule. False cysts may be inhomogeneous due to intralesional debris. On CT, wall calcifications are seen in 50% of cases (
Fig. 8
False cyst. Contrast-enhanced CT image shows a cystic unilocular, hypodense splenic mass with wall calcifications (arrow).

BENIGN TUMOURS
Haemangioma
Haemangiomas are vascular proliferations composed of channels filled with slow-flowing blood.(1) Depending on the size of these channels, haemangiomas are divided into capillary and cavernous types.(3) On US, a capillary haemangioma appears as a hyperechoic mass, whereas a cavernous haemangioma is heterogeneously hypoechoic, sometimes with calcifications or multiple cystic areas.(18) Abundant blood flow may be demonstrated with colour Doppler. On unenhanced CT, most haemangiomas are iso- to hypodense relative to normal splenic tissue. After contrast administration, cavernous haemangiomas show diffuse mottled or peripheral nodular enhancement extending toward the centre.(19) Capillary haemangiomas show homogeneous contrast enhancement.(1) Calcifications, either peripheral curvilinear or mottled centrally, may occur (
Fig. 9
Cavernous haemangioma. (a) Axial unenhanced CT image shows an amorphous calcification anterior to the spleen (arrow). Axial T1-W images after intravenous administration of gadolinium contrast in the (b) arterial; (c) portovenous; and (d) delayed phases show the calcification to be centrally located in a rounded lesion, representing a cavernous haemangioma. Note the typical irregular peripheral enhancement in the arterial phase, extending in a centripetal manner during the portovenous phase, and pooling in the delayed phase.

On MR imaging, haemangiomas are either hypo- or isointense on T1-WI and heterogeneously hyperintense on T2-WI.(3) A high signal intensity on T1-WI suggests the presence of subacute haemorrhage or proteinaceous content.(16) Superimposed infarction and thrombosis in larger haemangiomas may cause a variable MR imaging appearance.(20) In the latter case, differentiation from malignant disease may be difficult. As on CT, cavernous haemangiomas show peripheral enhancement extending toward the centre (Figs.
Lymphangioma
Lymphangiomas are malformations of the lymphatic system that consist of various sized cystic dilatations, containing lymph. Multiple lymphangiomas may be part of systemic lymphangiomatosis. Three histologic subtypes have been identified, depending on the size of the lymph channels, namely capillary, cavernous and cystic lymphangiomas.(18)
On US, typical lymphangiomas are multilocular, thin-walled, hypoechoic cyst-like lesions of different sizes, located close to the splenic capsule. Hyperechoic septa and intralocular echogenic debris may be detected.(18) On CT, tiny curvilinear peripheral calcifications may be occasionally identified. There is no contrast enhancement.(19) On MR imaging, multilocular lesions are homogeneously hyperintense on T2-WI and hypointense on T1-WI (
Fig. 10
Lymphangiomatosis. (a) Axial T2-W MR image shows multiple, multilocular cyst-like lesions (arrowheads). (b) T1-W MR image shows the cystic lesions appearing hypointense (arrowheads). (c) T1-W MR image after administration of gadolinium contrast, in the delayed phase, shows enhancement of the septa.

Littoral cell angioma
Littoral cell angioma represents a very rare benign vascular splenic tumour composed of anastomosing vascular channels.(18) Most patients are asymptomatic. The US appearance varies from splenomegaly with a mottled echotexture to multiple solid iso-, hypo- or hyperechoic nodules (
Fig. 11
Littoral cell angioma in a 35-year-old woman with anaemia and thrombocytopenia. (a) Axial US image of the spleen shows splenomegaly with a focal heterogeneous hyperechoic mass. (b) Axial contrast-enhanced CT image in the portovenous phase confirms an enlarged spleen, containing an irregularly delineated heterogeneous enhancing lesion (asterisk).

MALIGNANT TUMOURS
Lymphoma
Both Hodgkin and non-Hodgkin lymphomas represent the most common malignant neoplasms of the spleen.(14) Primary and secondary splenic involvement may occur. Primary involvement is rare, representing less than 1% of all lymphomas, most of which are non-Hodgkin lymphomas.(18) In secondary involvement, extrasplenic disease such as retroperitoneal lymph node enlargement often suggests the correct diagnosis.(19)
US of splenic lymphoma has a variable imaging appearance, ranging from a normal appearance, homogeneous or heterogeneous splenomegaly, multiple small (miliary) hypoechoic nodules (
Fig. 12
Multifocal splenic involvement in lymphoma. (a) US image shows several small hypoechoic splenic deposits in a patient with histologically proven mantle cell lymphoma (arrows). (b) Axial contrast-enhanced CT image acquired during the portovenous phase shows multiple low-attenuation lesions within an enlarged spleen (arrowheads). Variation in the size of lesions is more indicative of lymphomatous involvement rather than multifocal abscesses.

Fig. 13
Splenic involvement in lymphoma. (a) Axial contrast-enhanced CT image acquired during the arterial phase shows a large heterogeneous lesion (asterisk). (b) Axial T1-W image obtained after the administration of gadolinium contrast in the arterial phase shows viable (black arrow) and necrotic (white arrow) parts of the tumour. Note also that the normal parenchyma is almost entirely replaced by the lesion.

Metastasis
Splenic metastases are seen in 2%–9% of patients with metastatic end-stage cancer.(14) Haematogeneous spread from the breast, lung, ovary, stomach, prostate carcinoma and cutaneous melanoma are most common.(15) Evidence of metastatic spread, such as metastasis to the liver or other organs, is usually present. Peritoneal implants on the surface of the spleen are frequently seen in patients with primary tumours of the ovary, adenocarcinoma of the gastrointestinal tract and pancreatic cancer (
Fig. 14
Peritoneal splenic implant metastases in a patient with metastatic ovarian carcinoma. Axial contrast-enhanced CT image in the portovenous phase shows two well-circumscribed polylobulated and hypodense lesions on the dorsal surface of the spleen (white arrows). Note two more similar lesions on the dorsal surface of the liver (black arrows).

On US, metastases appear hypoechoic and occasionally mixed or hyperechoic. Cystic change is seen when necrosis occurs or due to the mucinous nature of the primary tumour (e.g. ovarian carcinoma).(19) On CT, splenic metastases appear as well-delineated, low-attenuation cystic or solid masses.(15) Calcification is unusual, unless the primary tumour is a mucinous adenocarcinoma. Most lesions show peripheral or septal enhancement (
Fig. 15
Splenic metastatic disease from lung carcinoma. Axial contrast-enhanced (a) CT and (b) T1-W images show two low-attenuation lesions with subtle peripheral enhancement (white arrow). Note a similar lesion posteriorly in the liver (black arrow), representing a liver metastasis.

Angiosarcoma
Splenic angiosarcoma is the most common malignant primary vascular neoplasm of the spleen.(20) Symptoms include weight loss, abdominal pain, malaise, fever, a palpable abdominal mass and hypovolemic shock due to splenic rupture.(21) On imaging, splenic angiosarcoma manifests as a poorly circumscribed heterogeneous mass, with internal areas of haemorrhage and necrosis.(21) Heterogeneous signal before and after contrast administration reflects the haemorrhagic nature of the tumour.(14) Differentiation from a large haemangioma with superimposed infarction and thrombosis may be difficult.(20)
SPLENIC INFARCTION
Splenic infarcts are common and can be of either arterial or venous origin. Arterial infarction occurs secondary to occlusion of the splenic artery (‘global infarction’) or one of its noncommunicating branches (‘segmental infarction’).(2) Thrombosis of the splenic sinusoids may cause venous infarction.(16) Complications of infarcts include splenic rupture, splenic pseudocyst formation, haemorrhage and superimposed infection with abscess formation.(3)
On US, acute splenic infarcts typically appear as wedge-shaped hypoechoic lesions pointing toward the splenic hilum.(1) On non-enhanced CT, infarcts are poorly visualised. After intravenous iodine contrast administration, the typical imaging findings are peripheral, wedge-shaped non-enhancing defects. However, this typical appearance is only present in less than half of all acute splenic infarcts.(2) In less typical cases, infarcts may mimic other splenic lesions, including abscesses or tumours, requiring clinical correlation, or if necessary, percutaneous fine-needle aspiration biopsy.(13) When the entire spleen is infarcted, it results in diffuse splenic hypodensity, leaving a residual rim of enhancing capsule supplied by small capsular vessels. This is known as the ‘rim sign’.(13) Chronic infarcts decrease in size, resulting in fibrotic retraction of the splenic capsule (
Fig. 16
Splenic infarction in a 79-year-old man with known atrial fibrillation. Coronal contrast-enhanced CT image shows a well-demarcated, wedge-shaped region of decreased enhancement with parenchymal loss and retraction of the splenic capsule, indicating the chronic nature of the infarction (arrow).

Fig. 17
Autosplenectomy in an adult with sickle cell disease. Axial contrast-enhanced CT image shows a small, shrunken spleen, with diffuse calcifications due to repeated micro-infarctions (arrow).

INFECTIOUS DISEASES
Splenic abscess is a rather uncommon lesion.(23) However, its incidence is increasing due to the widespread use of immunosuppressive drugs and chemotherapy. Splenic abscesses may be pyogenic, parasitic, fungal or tuberculous.(23)
Pyogenic abscesses
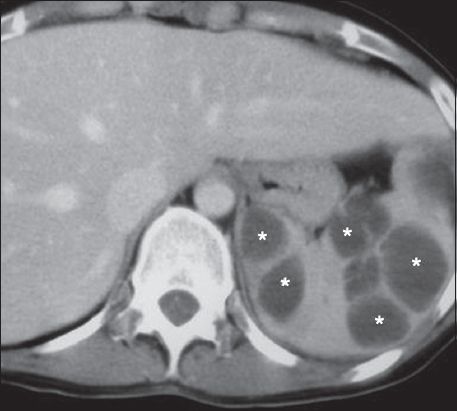
On US, abscesses appear as ill-defined hypo- or anechoic lesions, depending on the degree of proteinaceous fluid and necrosis. Debris, fluid levels and internal septations of varying thickness may be seen.(3) Intralesional gas, causing echogenic foci with ‘dirty’ shadowing, is highly suggestive of pyogenic infection, although the majority of splenic abscesses do not contain air.(3) On CT, pyogenic abscesses are irregularly marginated lesions, with inhomogeneous low attenuation (
Fig. 18
Pyogenic abscesses. Axial contrast-enhanced CT image acquired during the portal-venous phase shows multiple irregularly marginated non-enhancing lesions (asterisks).
Fungal abscesses
Fungal abscesses occur most commonly in immunocompromised individuals.(15) The most common infecting organisms are Candida albicans, Aspergillus fumigatus and Cryptococcus neoformans.(3) Fungal abscesses are typically multifocal and only a few millimetres in diameter.(19) On US, they are seen as lesions with a ‘target’ or ‘bull’s-eye’ appearance due to a central hyperechoic inflammatory core surrounded by hypoechoic fibrotic tissue.(3) The hyperechoic portion may become partially necrotic and hypoechoic, resulting in a ‘wheel-in-a-wheel’ pattern.(3) CT typically demonstrates multiple low-attenuation lesions, usually 5–10 mm in size (
Fig. 19
Fungal abscesses. Axial contrast-enhanced CT image acquired during the portal-venous phase shows multiple small non-enhancing splenic foci (white arrows). Note also multiple similar small non-enhancing foci in the liver, representing fungal liver abscesses (black arrows).

Tuberculous infection
There are two major morphological subtypes of hepatosplenic tuberculosis – micronodular and macronodular.(25) The micronodular (or miliary form) is the most common. On US, the micronodular form of hepatosplenic tuberculosis usually presents as a hyperechoic splenomegaly (‘bright spleen’). Rarely, multiple tiny hypoechoic and occasionally, hyperechoic focal lesions, are seen (
Fig. 20
Splenic tuberculosis. (a) Sagittal US image shows an enlarged spleen with multiple hypoechoic nodules of different sizes (arrows). (b) Contrast-enhanced CT shows widespread hypo-enhancing nodules, representing miliary splenic tuberculosis (arrowheads).

Although micronodular lesions are usually below the resolution of CT, tiny low-density foci are occasionally seen throughout the spleen (
INFLAMMATORY DISEASE
Sarcoidosis
Sarcoidosis is a multisystem granulomatous disease of unknown aetiology that is histologically characterised by multiple nonspecific non-caseating granulomas.(3) The lung, mediastinal and hilar lymph nodes are most frequently affected. The spleen is involved in up to 59% of cases.(3) On US, splenic sarcoidosis most commonly presents as a homogeneous enlarged spleen, with associated lymphadenopathy in the splenic hilum and retroperitoneum. A nodular form of involvement is seen in only 15% of cases.(21) These nodules may be detected as discrete hypoechoic lesions of variable sizes, ranging from 1 mm to 3 cm (
Fig. 21
Splenic sarcoidosis. (a) US image shows a normal-sized spleen with inhomogeneous echotexture and innumerable small hypoechoic lesions (arrows). (b) Coronal contrast-enhanced CT image acquired during the portovenous phase in a 50-year-old man shows multiple well-defined nodules of decreased enhancement throughout the spleen, representing small non-caseating granulomas (black arrows). Note the mediastinal and hilar adenopathies, which provide clues to the diagnosis (white arrows).

SPLENOMEGALY
Splenomegaly is a common nonspecific manifestation of many disorders.(3) The most common cause is portal hypertension, often associated with liver cirrhosis.(13) Different imaging modalities can be used to confirm splenomegaly and to search for extrasplenic manifestations of disease, providing clues to the correct cause.(1) Imaging may detect complications, including splenic rupture and infarction (
Fig. 22
Splenomegaly complicated with spontaneous rupture. Axial contrast-enhanced CT image shows free perisplenic fluid (asterisks) and an enlarged spleen with a heterogeneous area (arrows), which represents the area of laceration.

Gamna-Gandy bodies
Gamna-Gandy bodies are small haemorrhages in the spleen caused by different disorders, as summarised in
Table III
Aetiology of Gamna-Gandy bodies.

Fig. 23
Gamna-Gandy bodies in a 68-year-old man with liver cirrhosis and portal hypertension. (a) T1-W MR image obtained after administration of gadolinium contrast shows multiple hypointense foci throughout the spleen, representing haemosiderin (arrows). (b) Gradient-echo T1-W image shows the ‘blooming artefact’ due to the paramagnetic effect of haemosiderin. Note the nodular aspect of the liver parenchyma, which is compatible with cirrhosis.

SUMMARY
The spleen can be affected by a variety of conditions. US, CT and MR imaging are complementary tools for noninvasive characterisation and evaluation of splenic diseases. Many of these conditions can involve the spleen and have similar imaging manifestations. Therefore, imaging findings outside the spleen, along with the patient’s clinical history, may help to narrow the differential diagnosis. If uncertainty of a diagnosis persists, percutaneous biopsy may be considered.


