Abstract
INTRODUCTION
Intraoperative cell salvage (ICS) is an important aspect of patient blood management programmes. An ICS service was introduced at KK Women’s and Children’s Hospital, Singapore, from 2 May 2011 to 30 April 2013 to aid in the management of massive obstetric haemorrhage.
METHODS
With support from the Ministry of Health’s Healthcare Quality Improvement and Innovation Fund, a workgroup comprising obstetricians, anaesthetists and nursing staff was formed to develop training requirements, clinical guidelines and protocols for implementing ICS using the Haemonetics Cell Saver 5. Pregnant women with an anticipated blood loss of > 1,000 mL during Caesarean delivery, a baseline haemoglobin level of < 10 g/dL, rare blood types and who had refused donor blood were recruited to the service after obtaining informed consent.
RESULTS
A total of 11 women were recruited to the ICS service; the primary indications were placenta praevia and placenta accreta. Median blood loss in these 11 patients was 1,500 (range 400–3,000) mL. In four patients, adequate autologous blood was collected to initiate processing and salvaged, processed blood was successfully reinfused (mean 381.3 [range 223.0–700.0] mL). Median blood loss among these four patients was 2,000 (range 2,000–3,000) mL. No adverse event occurred following autologous transfusion. Mean immediate postoperative haemoglobin level was 8.0 (range 7.1–9.4) g/dL.
CONCLUSION
The implementation of an obstetric ICS service in our institution was successful. Future studies should seek to address the cost-effectiveness of ICS in reducing allogeneic blood utilisation.
INTRODUCTION
Management of postpartum haemorrhage (PPH) with the use of allogeneic blood transfusion is associated with high costs and complications, including errors in administration and infections.(1) In women with rare blood types or autoantibodies, it is logistically challenging to obtain appropriately matched blood in a timely manner during labour and delivery. Cell salvage is the process whereby a patient’s shed blood is collected, washed and processed for reinfusion. Salvaged red cells, which have a haematocrit (HCT) level of 40%–60%, are superior to banked blood in oxygen transport and viability, due to higher levels of 2,3-diphosphoglyceric acid and adenosine triphosphate.(2) While its use in cardiac and liver surgeries has been established, intraoperative cell salvage (ICS) was only endorsed for use in the obstetric population in 2005 by the National Institute for Health and Care Excellence,(3) the Obstetric Anaesthetists’ Association,(4) the Association of Anaesthetists of Great Britain and Ireland(4) and the American College of Obstetricians and Gynecologists.(5) We herein describe our clinical experience and highlight the lessons learnt in the implementation of an obstetric ICS service in KK Women’s and Children’s Hospital, Singapore, a tertiary obstetric hospital.
Our institution provides tertiary obstetric care for approximately 11,000–12,000 deliveries a year, with an overall Caesarean section rate of about 30%. Its incidence of PPH (defined as blood loss > 500 mL after vaginal delivery or > 1,000 mL after Caesarean delivery) is about 5% of deliveries. Up to 0.2% of deliveries may be complicated by massive haemorrhage (defined as the need for replacement of > 50% whole blood volume within four hours). An institution transfusion protocol is in place to guide transfusion therapy during PPH; it includes the activation of a massive transfusion protocol as well as the use of uterotonic agents and prompt surgical/radiological intervention to achieve haemostasis and a postresuscitation haemoglobin (Hb) level of at least 7 g/dL. A recent internal audit showed that 5% of the women in our institution required allogeneic blood transfusions. These transfusions took place predominantly during the peripartum period and were usually associated with Caesarean deliveries. Assuming compliance with the institution’s transfusion protocol, this suggests a high red cell transfusion rate among parturients. High red cell utilisation, coupled with the increasing evidence supporting the safety of obstetric cell salvage, served as an impetus for implementing an obstetric cell salvage service in our institution. Although autologous blood transfusion has been an important focus in patient blood management programmes worldwide, predelivery autologous blood harvesting is not a practical option due to the prevalence of thalassaemia in our population.(6) ICS, on the other hand, remains a practical option to obtain autologous blood from women undergoing Caesarean delivery.
METHODS
An obstetric ICS service was implemented in our institution for over two years, from 2 May 2011 to 30 April 2013, with the long-term aim of reducing allogeneic blood utilisation. Ethics approval from the institutional research board was sought but deemed unnecessary for quality assurance studies on established clinical practice.
With support from the Ministry of Health’s Healthcare Quality Improvement and Innovation Fund, a workgroup comprising obstetricians, anaesthetists and nursing staff was formed. An extensive literature search was first performed to gather current knowledge on the practice of ICS in both obstetric and non-obstetric populations. This was followed by the acquisition of necessary equipment through a stringent procurement process. Comprehensive guidelines and protocols were also developed to guide training, credentialling and service operation. They included the criteria for patient recruitment, roles and responsibilities of members of the ICS team, technical aspects of operation and methods to measure quality assurance.
The Haemonetics Cell Saver 5 (Haemonetics Corp, Braintree, MA, USA) was acquired due to its established use in obstetrics. The machine is fully automated, user-friendly and has separate disposable components for blood collection and processing. A team of three anaesthetic nurses (of senior staff nurse or nurse-clinician grade) was designated as the operator of the Haemonetics Cell Saver 5. The vendor provided training in the form of lectures and practical sessions over a span of two months. For the purpose of accreditation, we adopted the training and assessment criteria recommended by the United Kingdom Cell Salvage Action Group.(7) Upon achieving competence, the three anaesthetic nursing staff operated the Haemonetics Cell Saver 5 as technicians, with on-site support from the vendor for two years.
To gain the ground staff’s acceptance of the use of ICS, a familiarisation phase was initiated. In this phase, salvaged blood from the first two patients recruited to the service was processed and analysed at the laboratory for quality assurance, without reinfusion. Specifically, the salvaged, processed blood was passed through a leucocyte-depletion filter to simulate the reinfusion process, then collected for quantification of fetal red cells, alphafetoprotein (AFP), HCT and serum potassium. This was done to provide an indication of the quality of the salvaged blood, in the hope that the results would assure obstetricians about the safety of ICS. Workflow processes were also refined during this phase.
Patient recruitment for ICS started in October 2011. Target patients were those who (a) had a planned Caesarean delivery with an anticipated blood loss of > 1,000 mL (due to a morbidly adherent placenta, placenta praevia or risk factors for uterine atony), a preoperative haemoglobin (Hb) level of < 10 g/dL and rare blood types, and had refused donor blood; or (b) had a planned exploratory laparotomy following vaginal or Caesarean delivery due to postpartum haemorrhage.
ICS is contraindicated in the presence of Gelfoam®, Surgicel™, hypotonic or hypertonic solutions, hydrogen peroxide, iodine, lactated Ringer’s solution for peritoneal wash, bowel contents, fat, urine and tumour cells. At the start of the service, only surgeries performed during office hours were considered for ICS. A patient information sheet covering the benefits and risks of cell salvage was used to aid preoperative consent-taking. All the women recruited consented to ICS. For the purpose of quality assurance, data pertaining to ICS was recorded on a predesigned, anonymised form, including: indications for Caesarean section and ICS, baseline and postoperative Hb levels, estimated intraoperative blood loss, volume of salvaged autologous blood processed and reinfused, units of additional banked blood transfused, and the occurrence of any adverse events.
For all recruited patients, the Haemonetics Cell Saver 5 was first set up for collection. A minimum blood volume of 800 mL is required before the processing phase can be initiated. To save costs, the processing circuit was set up only when the minimal blood volume had been collected. It took only ten minutes for an experienced user to set up and prime the cell saver for collection. To avoid haemolysis, the suction pressure of the large-bore Yankauer aspirator catheter was limited to < 190 mmHg. In line with current practice, we used two suction catheters.(8,9) A ‘clean’ catheter was used for suctioning blood from the point of skin incision, up to and before the uterine incision. This was replaced by another (‘dirty’) catheter for suctioning blood that might be contaminated with amniotic fluid following uterine incision and rupture of amniotic membranes. The contents of the ‘dirty’ catheter were subsequently discarded. When all traces of amniotic fluid had been removed, the ‘dirty’ catheter was removed and blood salvage was resumed using the ‘clean’ catheter.
All processed, salvaged blood was reinfused via a leucocyte-depletion filter (LeukoGuard RS, Pall Medical, CA, USA) that is effective in removing 99% of leucocytes and 82% of lipid particles.(10) Each filter has a capacity of 450 mL. As leucocyte-depletion filters cannot distinguish between maternal and fetal red cells, Rhesus-negative mothers who gave birth to Rhesus-positive babies received an appropriate dose of anti-D immunoglobulin after delivery. Reinfusion of salvaged blood was done according to standard blood transfusion practice: the blood packs were labelled with the patient’s particulars, the blood collection date and time, as well as the expiry date and time. The time taken for the reinfusion process to be completed was set at < 6 hours so as to reduce microbial contamination. A pressure cuff was not applied on the salvaged blood pack as it could cause air embolism.
RESULTS
Table I
Results of laboratory assays of salvaged blood from two patients recruited for the familiarisation phase.
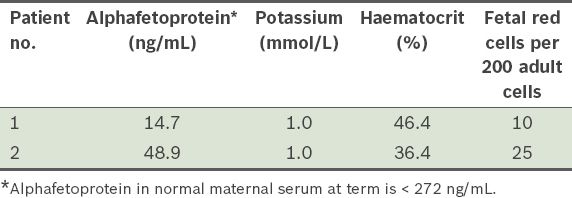
From September 2011 to April 2013, with the exclusion of four months of service disruption due to equipment failure, 11 women were recruited for the obstetric ICS service. The four-month disruption occurred after the cell saver failed to process blood in two patients and was recalled for repairs. Of the 11 women, nine were recruited for the indication of placenta praevia, one for the indication of a concurrent uterine fibroid in pregnancy and one due to the presence of multiple risk factors for uterine atony (
Table II
Pre- and postoperative details of patients recruited to the intraoperative cell salvage (ICS) service from September 2011 to April 2013 (n = 11).
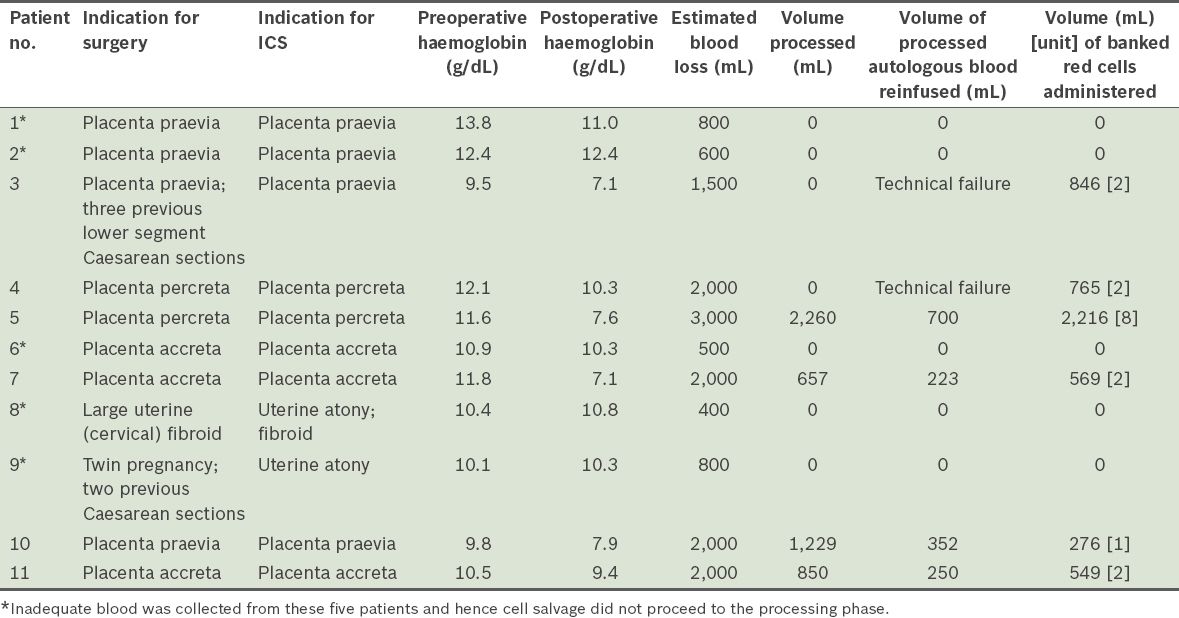
Median blood loss in the 11 women was 1,500 (range 400–3,000) mL. In six of the 11 women, adequate blood was collected to initiate processing. However, due to the equipment failure affecting two of these six patients, only four patients had their salvaged blood processed and reinfused successfully. The median blood loss in these four women was 2,000 (range 2,000–3,000) mL. The mean blood volume of the blood returned to them was 381.3 (range 223.0–700.0) mL. The four women who received the reinfusion of salvaged blood were also administered allogeneic banked blood. The ratio of total autologous salvaged blood to total banked blood by volume transfused to them was 1:2.4 (or 1,525 mL:3,610 mL). No adverse events occurred following transfusion of the autologous or banked blood.
The immediate postoperative Hb values of the four women who received salvaged blood were 7.6 g/dL, 7.1 g/dL, 7.9 g/dL and 9.4 g/dL. These values are well within the institution’s post-transfusion target Hb of 7.0 g/dL. The patient with the postoperative Hb of 9.4 g/dL received 250 mL of salvaged blood and two units of banked blood. In retrospect, she could have received only one unit of banked blood to achieve the post-transfusion target of 7.0 g/dL.
DISCUSSION
This report describes the clinical experience of introducing an obstetric ICS service in a tertiary hospital and the lessons learnt. Implementation of the service was done cautiously and strategically, using a phased approach. This was necessary to address the safety concerns related to amniotic fluid embolism and Rh isoimmunisation. Other hospitals have similarly adopted a phased approach to implementation, with a quality assurance and familiarisation phase preceding the introduction of an ICS service.(8) At an Australian maternity unit, 25 women recruited to the ICS service had their salvaged, processed blood tested for quality assurance without reinfusion, prior to service implementation.(8) Only two women were included in the familiarisation phase in the present study as there is ample evidence in the literature to demonstrate the safety of ICS in obstetrics (i.e. there was no need to replicate the quality assurance test on a large scale).(11-13)
Two important issues unique to obstetric cell salvage were addressed in previous studies: amniotic fluid embolism and Rh isoimmunisation.(14,15) Theoretically, there is a risk that the salvaged blood may be contaminated with amniotic fluid and hence elicit amniotic fluid embolism when reinfused into the maternal circulation. A growing body of evidence now suggests that the pathophysiological basis of amniotic fluid embolism is an anaphylactic mechanism,(14) as fetal squames have been isolated from the pulmonary circulation of otherwise-normal parturients.(15) Modern cell savers remove most particulate contaminants, and the use of discrete suction catheters and leucocyte-depletion filters during reinfusion enhances the safety of the procedure. The problem of Rhesus isoimmunisation of the Rhesus-negative mother can be managed by performing the Kleihauer-Betke test and administering an appropriate dose of anti-D immunoglobulin.
Based on an internal audit, 5% of the women in our institution received allogeneic blood in the antepartum or peripartum period in a year. This figure likely reflects our institution’s high clinical burden of women with PPH, as it is a tertiary referral centre for high-risk obstetric patients. With uterine blood flow reaching 800 mL/min at term, the rate of bleeding in an obstetric patient is comparable to that of a multitrauma patient. ICS is a means of reducing allogeneic blood transfusion during Caesarean deliveries. Based on the present study, six of 11 women recruited were amenable to this blood conservation technique. The total volume of processed autologous blood returned was 1,525 mL, while the total volume of allogeneic banked blood administered to the four women who had successful transfusions of processed autologous blood was 3,610 mL (
Setting up an ICS service is costly due to high equipment and manpower costs. To reduce manpower costs, we re-engineered staff roles and facilitated creative staff rostering. Anaesthetic staff nurses who were already operating room staff were trained as cell saver technicians. For each case of ICS managed in the operating room, two anaesthetic staff nurses were deployed; one would be in charge of operating the cell saver, while the other would assist the attending anaesthetist in providing anaesthesia care. By exercising flexibility in the nurses’ job scopes and creativity in staff deployment, we were able to save the cost of employing additional full-time staff to function as cell saver technicians.
The long-term goal of the ICS service, as an important aspect of patient blood management programmes, was to reduce allogeneic blood transfusion. Such a reduction may help to justify the costs associated with the implementation of an ICS service. Hence, a cost comparison between salvaged and allogeneic blood is necessary to determine the economic benefits. The cost of processing donor blood has soared in the last decade due to the stringent testing required to detect the blood-borne antigens of acquired immune deficiency syndrome, hepatitis and Creutzfeldt-Jakob disease. One standard unit of donor red cells, before government subsidy, may range from SGD 150 to over SGD 200, depending on the requirements for further processing. As for salvaged blood, two studies have suggested that the volume of ICS cases has direct impact on its cost.(16,17) In a cost analysis study of a cell salvage service that was based on an annual case load of 2,500,(16) a unit of autologous red cells processed by cell salvage cost USD 89.46, while allogeneic red cells cost USD 200. With high case volume, manpower and equipment costs are distributed over a larger patient-consumer population, which then reduces the cost for individuals.
There may be indirect costs associated with allogeneic blood transfusion related to the management of transfusion-related complications, such as transfusion-related acute lung injury, transfusion reactions and transmission of blood-borne antigens. Management of these complications can be costly for both the hospital and the patient. Although the specific risks associated with the transfusion of autologous salvaged blood are unknown, they are estimated to be low, at 0.027% (vs. 0.140% for allogeneic blood).(18) Adverse reactions to salvaged blood are rare and include nonimmune haemolysis, air embolism and febrile nonhaemolytic transfusion reactions.
In the present study, we were unable to evaluate the effectiveness of the ICS service in reducing allogeneic blood utilisation due to the small number of women recruited to the service. A 2006 Cochrane Collaboration meta-analysis found that the use of cell salvage reduced the rate of exposure to allogeneic blood transfusion by 38% in general surgical patients (relative risk 0.62, 95% confidence interval 0.55–0.70), with an average saving of 0.67 units per patient.(19) To increase the cost-effectiveness of ICS in our institution, there is a need to increase the volume of ICS cases. This can be achieved by extending the service to after office hours, as a greater proportion of emergency Caesarean deliveries occur at that time. However, this would require after-hours staff to be trained as technicians. Another means to increase the ICS caseload is to extend the service to other surgical procedures that may involve intraoperative haemorrhage, such as major non-cancer operations as well as trauma-related, spine and cardiac surgeries.
Other measures that can be taken to improve the cost-effectiveness of ICS include the use of strategies to improve red cell recovery and yield in cell salvage. The surgeon collecting the shed blood should be trained in the proper technique of aspiration to avoid causing haemolysis with excessive surface skimming. Red cells on gauze and swabs could be recovered by soaking them in sterile normal saline and using gentle compression to expel the red cells(20) before suctioning for collection. When blood loss is rapid, suction pressures can be increased transiently without affecting red cell quality. Lastly, to reduce wastage, disposable sets for processing should only be set up when at least 800 mL of shed blood has been collected.
In this study, we described the successful introduction of an ICS service in a tertiary referral centre. Cell salvage, an important focus in comprehensive patient blood management programmes, should be integrated into the protocol for managing massive obstetric haemorrhage. To maintain the cost-effectiveness of the service, there is a need to increase the case volume. This can be done by providing ICS service after hours and extending the service to other surgical procedures in which major haemorrhage is likely to occur. Future studies should seek to address the effectiveness of an obstetric ICS service in reducing allogeneic blood utilisation.
ACKNOWLEDGEMENT
The project was supported by the Ministry of Health’s Healthcare Quality Improvement and Innovation Fund.


