Abstract
Clinical trials have established the benefits of implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy (CRT) in the treatment of heart failure patients. As adjuncts to guideline-directed medical therapy, ICDs confer mortality benefits from sudden cardiac arrest, while CRT reduces mortality, hospitalisation rates and improves functional capacity. This review discusses the use of ICDs and CRT devices in heart failure management, outlining the evidence supporting their use, indications and contraindications.
INTRODUCTION
In Singapore, the age-standardised incidence rate of acute myocardial infarction increased from 208.9 per 100,000 in 2007 to 221.2 per 100,000 in 2013. During the same period, the age-standardised mortality rate from acute myocardial infarction decreased from 40.8 per 100,000 to 26.0 per 100,000, with heart failure being one of the top complications. Consequently, the overall heart failure disease burden in Singapore has increased over the years to make heart failure one of the most common cardiac causes of hospital admission.(1)
In addition to guideline-directed medical therapy (GDMT), implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy (CRT) have been reported to be effective adjuncts in heart failure management. These devices have been shown to be useful for the prevention of sudden cardiac death (SCD) and progressive pump failure, the two main causes of cardiac death in patients with left ventricular (LV) dysfunction. This review discusses ICDs and CRT devices and the roles they play in heart failure treatment.
Implantable cardioverter defibrillator
Patients with LV dysfunction are at an increased risk of SCD secondary to ventricular tachyarrhythmias. The risk of SCD increases with decreasing LV ejection fraction.(2-6) Those who have experienced previous sustained ventricular tachyarrhythmias or unexplained syncope are at the greatest risk of SCD. The ICD is a device capable of detecting and terminating ventricular tachyarrhythmias. It consists of leads attached to a pulse generator that houses the batteries, microprocessors and capacitors. An ICD pulse generator is usually implanted subcutaneously in the left anterior chest wall under local anaesthesia and conscious sedation, with leads introduced into the cardiac chambers via the subclavian or cephalic vein. A single-chamber ICD has a single defibrillator lead implanted in the right ventricle (
Fig. 1
Radiograph shows a single-chamber implantable cardioverter defibrillator with a thick radiopaque shocking coil in the right ventricular defibrillator lead.
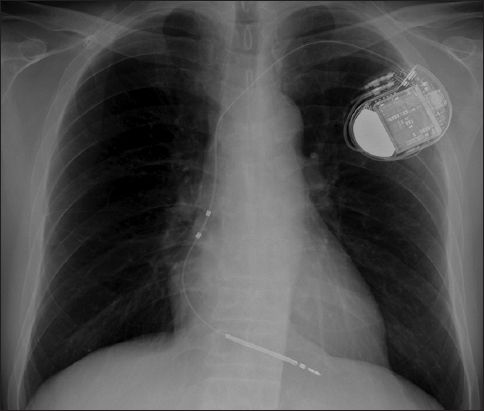
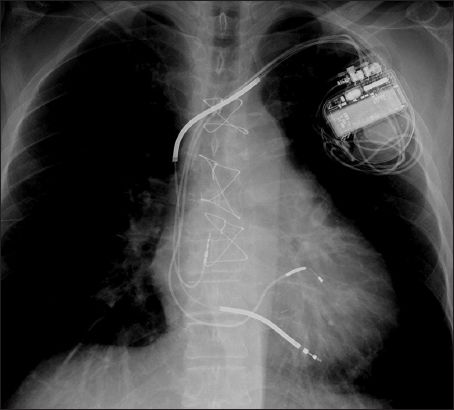
Fig. 2
Radiograph shows a biventricular defibrillator or cardiac resynchronisation therapy-defibrillator with a right atrial lead and right ventricular defibrillator lead, as identified by the presence of shocking coils and a left ventricular lead coursing through the coronary sinus to pace the left ventricle.
In large prospective trials for the secondary prevention of SCD, such as the Antiarrhythmics Versus Implantable Defibrillators study, Cardiac Arrest Study Hamburg and Canadian Implantable Defibrillator Study, ICD use resulted in relative risk reduction of up to 50% for arrhythmic deaths and 25% for all-cause mortality.(7-12) Studies evaluating the secondary prevention of SCD have consistently shown better survival with ICD therapy compared to anti-arrhythmic medications.(12)
The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) and Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) provided evidence supporting ICD use for the primary prevention of SCD in patients with LV dysfunction. Importantly, all patients who were studied had already been on at least 3–6 months of GDMT. In SCD-HeFT, patients had LV ejection fraction ≤ 35% with New York Heart Association (NYHA) Class II or III symptoms secondary to ischaemic or non-ischaemic causes. There was a 23% relative reduction in risk of death and 7% absolute reduction in mortality after five years of ICD use.(13) Patients who were randomised to the ICD arm had 60% reduction in SCD.(14) The reduction in all-cause mortality was independent of ischaemic or non-ischaemic aetiologies of cardiac failure. In MADIT-II, patients had LV ejection fraction ≤ 30% and were at least 30 days post-myocardial infarction. All-cause mortality was reduced by 31% with ICD use; patients whose index heart attack was more distant from the point of randomisation derived more benefit from ICD therapy.(15,16)
For patients with LV dysfunction secondary to non-ischaemic aetiologies, the DEFibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) and SCD-HeFT trials supported the use of ICD for the primary prevention of SCD. The DEFINITE trial also used an LV ejection fraction cut-off criterion of ≤ 35%. Both trials showed decreases in all-cause and arrhythmic mortality.(13,17) Desai et al performed a meta-analysis of five primary prevention trials involving 1,854 patients with non-ischaemic heart failure and reported a significant 31% reduction in total mortality with ICD use.(18)
Current evidence does not support ICD therapy in patients with NYHA Class IV end-stage cardiac failure or an estimated life expectancy of less than one year. ICDs are also not recommended for the primary prevention of SCD in NYHA Class I patients and cases of heart failure with preserved LV ejection fraction.(19) Older patients are underrepresented in ICD trials, where the average patient age is 65 years or less.(20) Trials for the secondary prevention of SCD revealed no benefit in patients aged 75 years or older and a meta-analysis of trials for the primary prevention of SCD suggested that ICD therapy was less efficacious in older patients.(21,22) Patients with repeat hospitalisation for heart failure, especially when coupled with chronic kidney disease, may also benefit less from ICD therapy.(23)
With the exception of MADIT-II, most other trials evaluating ICD efficacy involved only single-chamber devices or very few dual-chamber ones.(24) Studies evaluating the clinical superiority of dual- versus single-chamber ICDs have shown mixed results. Some reports suggested that the dual-chamber ICD provided clinical benefits in arrhythmia differentiation, prevention of inappropriate ICD therapy and patients who required pacing for bradyarrhythmias.(25-29) However, Dewland et al pointed out that complications were more frequent during the implantation of dual- versus single-chamber ICDs (3.17% vs. 2.11%; p < 0.001), with a higher in-hospital mortality (0.40% vs. 0.23%; p < 0.001).(24)
Taking into account existing best evidence, American and European guidelines(19,30) recommend the following criteria as Class I indications for ICD implantation in patients with LV dysfunction:
Documented ventricular fibrillation or haemodynamically unstable sustained ventricular tachycardia with no identifiable reversible causes; on GDMT; and with good functional capacity and life expectancy of more than one year.
Ischaemic or non-ischaemic LV dysfunction with ejection fraction of ≤ 35%; and NYHA Class II or III status, with good functional capacity or life expectancy of more than one year (for patients with ischaemic LV dysfunction, the index acute myocardial infarction should be 40 days or longer). European guidelines further recommend that patients should be on at least three months of GDMT.
Ischaemic LV dysfunction and ejection fraction of ≤ 30%; NYHA Class I status; and at least 40 days post-myocardial infarction with good functional capacity and life expectancy of more than one year.
Conversely, ICD implantation is contraindicated in the following clinical situations:
Estimated life expectancy of less than one year and/or poor functional capacity.
Incessant ventricular tachyarrhythmias.
NYHA Class IV patients who fail to respond to GDMT and are not deemed suitable for cardiac transplantation or CRT.
Ventricular tachyarrhythmias that are amenable to surgical/catheter ablation or secondary to reversible causes.
Cardiac resynchronisation therapy
As heart failure progresses, electrical remodelling occurs and the QRS duration may be considerably prolonged in up to one-third of patients, resulting in a poorer outcome.(31) Intraventricular and interventricular mechanical dyssynchrony develop as a result of electrical remodelling and, in turn, negatively impact cardiac contractile performance. CRT or biventricular pacing confers benefits on such patients through improvements in ventricular contractility, functional mitral regurgitation, ventricular remodelling and overall LV ejection fraction. As it results in increased blood pressure, doses of GDMT may be further optimised with the potential for greater improvement.
Two randomised controlled trials (the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure [COMPANION] trial and the CArdiac Resynchronisation-Heart Failure [CARE-HF] trial) have shown that in patients with reduced LV ejection fraction, sinus rhythm, NYHA Class III and ambulatory Class IV symptoms, CRT decreases morbidity and mortality.(32,33) Both trials enrolled patients with QRS duration > 120 ms. All-cause mortality was reduced by up to 36%. In the COMPANION trial, CRT-defibrillator use, but not CRT-pacemaker, decreased SCD rate, while in the extended CARE-HF trial, which had a mean follow-up duration of 37 months, CRT-pacemaker use also decreased SCD by 46%. Both trials provided compelling evidence supporting the use of CRT in heart failure patients who have reduced LV ejection fraction; NYHA Class III or ambulatory Class IV symptoms; QRS duration > 120 ms; and, in particular, those with left bundle branch block (LBBB) morphology. Other trials, registries and meta-analyses subsequently reported findings corroborating those of the COMPANION and CARE-HF trials.(34-36) In a meta-analysis by Sipahi et al, CRT use was associated with a significant reduction in all-cause mortality or hospitalisation rates in patients with QRS duration > 150 ms, but not in those with a QRS duration of 120–150 ms.(37)
In patients with mild heart failure, two randomised controlled trials have shown the benefits of CRT over optimal GDMT. The Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) evaluated patients with LV ejection fraction < 30%, NYHA Class I or II symptoms, and QRS duration > 130 ms. It reported a 34% reduction in all-cause mortality or heart failure events with the use of CRT. After seven years of follow-up, patients with ICDs were compared to those with CRT-defibrillators and a significant reduction in mortality was found in patients with baseline LBBB.(38) The Resynchronisation-Defibrillation for Ambulatory Heart Failure Trial, which enrolled patients with LV ejection fraction < 30%, QRS duration > 120 ms or NYHA Class II or III symptoms, reported a significant 25% relative reduction in all-cause mortality when comparing patients with CRT-defibrillator and those with ICD.(39)
The Dual Chamber and VVI Implantable Defibrillator (DAVID) trial(40) and Mode Selection Trial(41) showed that right ventricular pacing was associated with an increased hospitalisation rate for heart failure, suggesting that CRT confers clinical benefits in patients with reduced LV function and a need for pacing.(42) In the DAVID trial, patients with a right ventricular pacing burden of > 40% had poorer outcomes. The Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block trial corroborated these findings with revelations that CRT was associated with a 26% decrease in primary composite end-point (total mortality, urgent heart failure care or an increase in LV end-systolic volume) when compared with right ventricular pacing in cardiac failure patients with conventional indications for pacing.(43)
Patients with permanent atrial fibrillation derive appreciably fewer clinical benefits from CRT.(44,45) However, long-term survival following CRT in atrial fibrillation patients who have undergone atrioventricular (AV) node ablation is similar to that of patients with sinus rhythm. AV node ablation ensures a high degree of biventricular pacing, which is essential for effective CRT.(46)
Elderly patients were underrepresented in the main randomised CRT clinical trials, whose participants had a mean age of 64–68 years.(31-33) However, there is published data suggesting that CRT is safe and effective for the elderly. A subgroup analysis of MADIT-CRT evaluating the degree of LV end-systolic volume change at 12 months follow-up among patients aged < 60 years, 60–74 years and ≥ 75 years found no significant differences between age groups.(47) Comparable findings were also reported in subgroup analyses involving patients from COMPANION,(32) CARE-HF(33) and several cohort studies.(48-50) Device-related complication rates did not differ across age groups. In a COMPANION subgroup analysis, there was no significant difference in survival rates between elderly and non-elderly patients.(32)
Current established Class I indications for CRT(51,52) include patients who fulfil the following criteria:
NYHA Class III or ambulatory Class IV symptoms; LV ejection fraction ≤ 35%; baseline LBBB; sinus rhythm; and QRS duration of ≥ 150 ms on optimal GDMT (European guidelines recommend at least three months of GDMT).
NYHA Class I or II symptoms; LV ejection fraction ≤ 30%; baseline LBBB; sinus rhythm; and QRS duration ≥ 130 ms on optimal GDMT (European guidelines recommend at least three months of GDMT).
Upgrade from a cardiac permanent pacemaker or ICD in a patient with LVEF ≤ 35%; high percentage of ventricular pacing; and NYHA Class III or ambulatory NYHA Class IV symptoms.
At this juncture, there is no evidence to support the use of CRT in patients with non-LBBB electrocardiography patterns, QRS duration < 120 ms or survival of less than one year. The Evaluation of Resynchronization Therapy for Heart Failure and Echocardiography Guided Cardiac Resynchronization Therapy (EchoCRT) trials provided evidence that in patients with mechanical dyssynchrony but narrow QRS duration (< 130 ms), CRT did not improve clinical outcomes or reverse LV remodelling, and instead increased mortality.(53,54)
Despite fulfilling the selection criteria, up to one-third of patients may not exhibit a response to CRT.(55) To date, there is no standard definition of CRT response. Some studies reported CRT response in terms of clinical parameters such as the heart failure clinical composite score, which combines all-cause mortality, heart failure hospitalisation rate, NYHA class and patient global assessment into an outcome measure.(31,56) Others evaluated response in terms of echocardiographic parameters such as ≥ 15% reduction in left ventricular end-systolic volume.(57,58) Published data revealed a discrepancy between clinical and echocardiographic responses, with more studies reporting clinical response than echocardiographic improvement.(59)
Reduced response to CRT has been associated with a high myocardial scar burden,(60) posterolateral(61) and mid-wall(62) scar location, extreme mechanical dyssynchrony,(63) severe right ventricular dysfunction, pulmonary hypertension, end-stage renal failure, and valvular heart disease. On the other hand, better CRT outcomes have been reported in female patients(64,65) and those with non-ischaemic cardiomyopathy.(62)
Efforts have been made to identify factors which may better predict response to CRT. The Predictors of Response to CRT trial(66) and EchoCRT study(54) revealed that echocardiographic measures of mechanical dyssynchrony did not reliably predict CRT response. Consequently, clinical guidelines on CRT do not recommend use of echocardiographic parameters for patient selection. The role of imaging in patient selection for CRT has shifted toward identification of optimal LV pacing sites. The Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy study(67) and Speckle Tracking Assisted Resynchronization Therapy for Electrode Region trial(68) reported that CRT outcomes may be improved by using echocardiography to identify late-activated segments for LV pacing. The use of cardiac magnetic resonance imaging to assess myocardial scar burden and avoid LV pacing at scarred areas also appears promising.(69,70) Trials evaluating AV and ventricular-ventricular (VV) optimisation after CRT implantation have largely yielded negative results. Compared to default settings, neither echocardiographic nor algorithm-based AV and VV optimisation confers long-term benefits.(71,72,73)
CONCLUSION
Despite the evidence supporting ICD and CRT use in heart failure management, the implantation rate remains low in Singapore and the rest of Southeast Asia, even in patients who fulfil the clinical indications as stipulated in current guidelines.(74) Among Asian patients, data on device efficacy appears inconsistent.(75,76) More research is therefore necessary to evaluate device efficacy in Asian heart failure patients and study the cost-effectiveness of such device therapy in the healthcare systems of the various Asian countries. Finally, it is important to raise awareness of the clinical benefits of ICD and CRT in heart failure treatment among healthcare professionals managing cardiac failure patients, so that they can initiate appropriate referrals for device implantation.


