Abstract
INTRODUCTION
Many institutions still perform routine chest radiography (CXR) after tube thoracostomies despite current guidelines suggesting that this is not necessary for simple cases. We aimed to evaluate the usefulness of routine CXR following ultrasonography-guided catheter thoracostomies for the detection of complications of symptomatic pleural effusions in hospitalised patients.
METHODS
This was a retrospective review of 2,032 ultrasonography-guided thoracostomies on hospitalised patients with symptomatic effusions at a single institution from April 2012 to May 2015. The aetiology of effusions was not systemically registered, but patient demographics, procedural details and clinical outcomes were collected. Data was analysed using descriptive statistics and chi-square test. Generalised estimating equation analysis was performed to assess the relationship between CXR findings and complications while controlling for age.
RESULTS
Out of 2,032 CXRs, 92.96% (n = 1,889) were normal, 5.81% (n = 118) showed pneumothorax and 1.23% (n = 25) showed catheter kinking. 99 pneumothoraces and 24 kinked catheters were detected in the first hour post procedure. 97.40% (n = 115) of patients with pneumothorax were stable or had minor complications, such as a vasovagal event. 0.20% (n = 4) of the cases had a serious complication following chest drain insertion, resulting in cardiovascular collapse. There was no significant relationship between CXR results and occurrence of complications (p = 0.244). Amount of fluid drained or side of insertion did not affect the clinical outcome.
CONCLUSION
Routine use of CXR after tube thoracostomy did not significantly change patient management, which was concordant with recent guidelines. Instead, adverse clinical outcomes or procedural factors should guide investigations.
INTRODUCTION
Small-bore catheters (7–14 Fr) have been gaining popularity in recent years for treatment of symptomatic effusions.(1) Current practice guidelines do not require chest radiography to be performed after simple pleural aspiration unless air was withdrawn, the procedure was difficult, multiple attempts were required, or the patient became symptomatic.(1) Several studies have also shown that the incidence of post-procedure complications of ultrasonography-guided drainage of pleural effusions is low,(1-7) with few clinically significant events that require urgent intervention.(8) Furthermore, it has been shown that the insertion of pigtail catheters results in low complication rates when compared to large-bore chest tubes.(1,9-11) It has been our department’s experience that small-bore catheter insertion under ultrasonography guidance results in accurate positioning and minimal complications in the setting of symptomatic pleural effusions. However, many institutions continue to routinely perform chest radiography following tube thoracostomy despite recommendations by existing guidelines. The objective of this study was to assess the value of routine chest radiography after ultrasonography-guided catheter thoracostomies for the detection of complications of symptomatic pleural effusions in hospitalised patients.
METHODS
This study was approved by the institutional ethics review board. A retrospective review of the radiology information system database at the Department of Diagnostic Radiology, Tan Tock Seng Hospital, Singapore, was performed. All ultrasonography-guided pleural drainage procedures performed by interventional radiologists at our department for hospitalised patients with symptomatic effusions from April 2012 to May 2015 were selected. Chest radiography was routinely performed at our institution after pleural drainage. The aetiology of effusions was not systemically registered. Data on patient demographics, side of tube insertion, size of tube used, amount of fluid drained, time interval of the radiograph from the completion of procedure, radiography findings, and clinical outcomes was collected.
During the 37-month study period, 2,050 ultrasonography-guided pleural drainages were performed on 1,539 patients. 18 pleural procedures on 13 patients were excluded, as post-procedure chest radiography was not performed. A total of 2,032 ultrasonography-guided catheter thoracostomies performed on 1,526 patients were thus included for analysis. Each thoracostomy was considered as a unit of analysis.
For the small-bore pigtail catheter insertion technique, catheters were inserted under direct ultrasonography guidance. First, the drainage site was determined by ultrasonography, identifying the main collection of pleural fluid. Local anaesthesia was administered into the chest wall. Subsequent insertion of an 18-G Surflo venula (Terumo, Tokyo, Japan) into the pleural cavity was performed under direct ultrasonographic guidance. After confirmatory aspiration of the pleural fluid, a J-tipped guidewire was inserted into the pleural cavity after removal of the stylet. Sequential soft-tissue dilators were used to dilate the tract before passing the small-bore pleural catheter of choice into the pleural cavity. The final catheter position was determined by ultrasonography before the catheter was finally secured with anchoring sutures to a stoma base plate. Up to 1 L of pleural fluid was hand aspirated in most cases before clamping the drainage catheter. The drainage catheter was immediately connected to an underwater seal. A post-procedure chest radiograph was obtained.
Data was analysed using descriptive statistics. Comparison between two groups was carried out using chi-square test. Generalised estimating equation statistical analysis was performed to assess the relationship between chest radiography findings and complications, while controlling for age. The analyses were performed using IBM SPSS Statistics version 22.0 (IBM Corp, Armonk, NY, USA).
RESULTS
There were 894 (58.58%) men and 632 (41.42%) women, with a mean age of 67 ± 14 (range 17–102) years. Pigtail catheters in the range of 8–14 Fr were used for pleural drainage. 99.61% (n = 2,024) of the catheters used were 10-Fr Navarre (Bard, NJ, USA) or ReSolve (Merit Medical, UT, USA) catheters. 89.22% of the post-procedure radiographs were obtained within an hour and 97.44% within four hours of the ultrasonography-guided catheter thoracostomy (
Table I
Time of chest radiography (CXR) after the procedure and findings (n = 2,032).
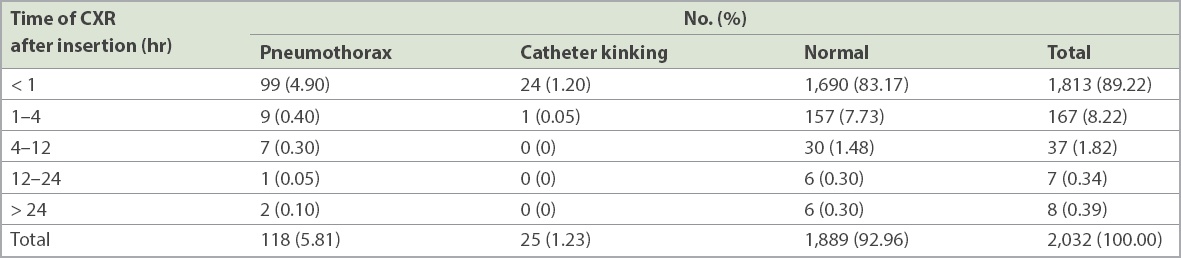
92.96% (n = 1,889) of chest radiographs were normal, while 5.81% (n = 118) showed pneumothorax and 1.23% (n = 25) had catheter kinking (
Table II
Chest radiography (CXR) findings and clinical outcomes (n = 2,032).
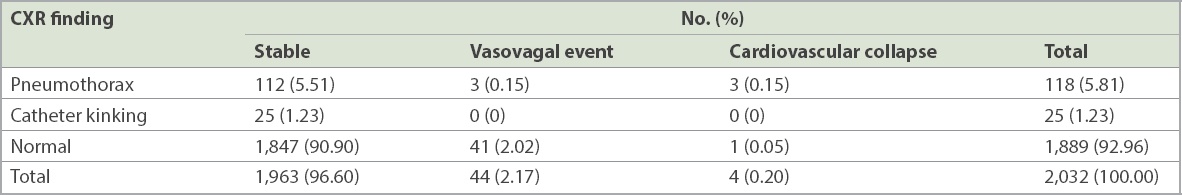
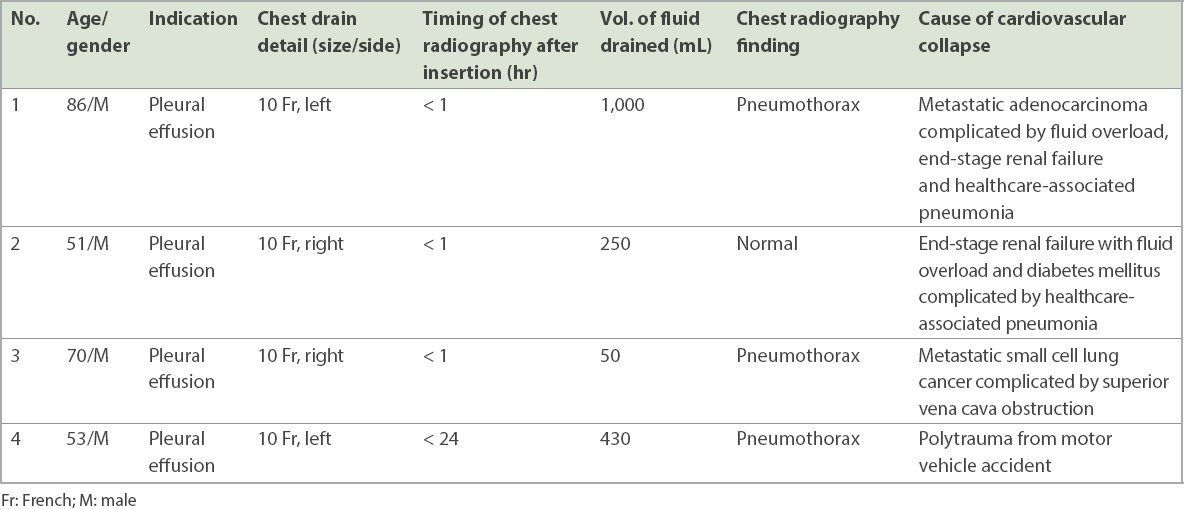
0.20% (n = 4) of the cases had a serious complication following chest drain insertion, with eventual cardiovascular collapse, as detailed in
Table III
Overall data of patients with cardiovascular collapse subsequent to chest drain insertion (n = 4).
60.68% (n = 1,233) of the procedures were performed by staff radiologists with at least five years of post-fellowship experience, and 39.32% (n = 799) were performed by radiology residents under supervision. Residents had a statistically significant higher rate of complications. Pneumothorax occurred in 7.51% (60/799) of procedures performed by residents, compared to 4.70% (58/1,233) of procedures performed by staff radiologists (p < 0.05).
During ultrasonography-guided pleural drainage, the amount of fluid drained was as follows: < 100 mL in 209 (10.29%) cases, 100–250 mL in 371 (18.26%) cases, 251–500 mL in 437 (21.51%) cases, 501–750 mL in 329 (16.19%) cases, 751–1,000 mL in 407 (20.03%) cases, 1,001–1,500 mL in 39 (1.92%) cases and > 1,500 mL in 5 (0.25%) cases. For 235 (11.56%) cases, the amount of fluid drained was not recorded in the procedural notes. 773 (38.04%) cases had drainage in the left hemithorax and 1,259 (61.96%) cases had drainage in the right hemithorax. There was no significant relationship between the volume of fluid drained (p = 0.10) or the side of drain insertion (p = 0.740) and the occurrence of complications.
Subgroup analysis of pleural procedures with kinked tubes (n = 25) demonstrated that the amount of fluid drained was as follows: < 100 mL in 8 (32.00%) cases, 100–250 mL in 8 (32.00%) cases, 251–500 mL in 2 (8.00%) cases, 501–750 mL in 2 (8.00%) cases and 751–1,000 mL in 3 (12.00%) cases. For 2 (8.00%) cases, the amount of fluid drained was not recorded in the procedural notes.
DISCUSSION
Ultrasonography-guided thoracocentesis(5,8,12) and tube thoracostomy(2,3,11,13) are safe procedures with few complications. A majority of complications in our patients were detected on post-procedure chest radiography performed within the first hour. However, these complications were not clinically significant, with no cardiovascular collapse noted. Suboptimal catheter placement with kinking will result in poor fluid drainage or resistance to aspiration, which may prompt further investigations. The kinked tubes were repositioned within 24 hours of detection. These tubes were initially assessed to have adequate flow on hand aspiration during the procedure. Movement of the tubes during patient transfer and transport could have occurred despite the anchoring suture, resulting in poor drainage in the wards. The pneumothoraces in patients who developed kinked tubes were small (less than 3 cm apex to cupola distance) and resolved after observation or oxygen therapy; these cases did not require invasive intervention.
Our findings suggest that instead of having routine chest radiography following ultrasonography-guided catheter thoracostomies, considerations about performing post-procedure chest radiography should be guided by procedural factors (e.g. withdrawal of air, difficult procedure and multiple attempts),(1) the patient’s clinical conditions and/or catheter performance. Unstable or deteriorating blood pressure, oxygen saturation or increased oxygen requirement should prompt further investigations. In patients who had cardiovascular collapse in our study, the detection of abnormalities did not predict impending collapse. Incidentally, in one patient who collapsed, the post-procedure chest radiograph was normal. Several authors(7,8,14,15) have arrived at similar conclusions that post-procedure chest radiography should be guided by clinical symptoms. Our findings also concur with the existing British Thoracic Society (BTS) guidelines on pleural disease.(1)
Varying amounts of pleural fluid were drained from patients in this study. In our practice, this is guided by the size of pleural effusion, whether the patient becomes symptomatic during initial drainage (either coughing or breathlessness), and whether resistance and poor drain performance was encountered during initial drainage. We generally adhere to BTS guidelines as part of our departmental practice, draining up to 1 L of pleural fluid in order to reduce the risk of re-expansion pulmonary oedema. 44 (2.17%) cases had over 1 L of pleural fluid drained during the procedure in our study. However, no documentation was available for these patients to account for the increased volumes of drainage. Some practitioners may drain more than 1 L for patients with very large recurrent symptomatic pleural effusions to the point of symptom relief, on a case-by-case basis.
Subgroup analysis of chest radiographs with kinked catheters revealed that 64.0% (16/25) had drainage volumes of less than 250 mL. This would suggest that in patients with small pleural effusions, it might be difficult to position the catheters in the optimal position due to less space for manipulation.
In our study, a majority of radiographs were normal. Among procedures with abnormal radiographs, nearly all patients were stable and abnormalities were clinically insignificant. For patients who had post-procedure cardiovascular collapse, the radiographs did not provide sufficient information to warn of the impending collapse. The patients who collapsed after catheter insertion were individually reviewed and shown to have severe comorbidities, such as metastatic lung cancer, severe pneumonia, end-stage renal failure with fluid overload, and polytrauma with severe injuries. These patients had cardiovascular collapse and died more than 24 hours post procedure.
It was also noted that procedures performed by residents had a statistically significant higher rate of complications. This may be due to prolonged procedure time, resulting in more manipulation. However, post-procedure chest radiography was still not warranted for such patients, as the number of pneumothoraces encountered in our study was not clinically significant.
In conclusion, this large-scale, single-centre retrospective study suggested that chest radiography findings following ultrasonography-guided catheter thoracostomies do not often change patient management, which is concordant with the 2010 BTS guidelines.(1) Instead, procedural factors and clinical outcomes such as unstable vital signs or poor drainage should guide further investigations in these hospitalised patients. Additional studies may be performed to evaluate reduction in radiation exposure, as well as associated cost savings if post-procedure radiographs were to be omitted for these patients.
ACKNOWLEDGEMENTS
We thank Ms Sun Bing and Ms Tang Xin from the Clinical Research and Innovation Office, Tan Tock Seng Hospital, Singapore, for their assistance with statistical analysis.


