CASE PRESENTATION
An 87-year-old woman presented to the emergency department with a one-day history of haematuria and fever. Significant past medical history included chronic left hydroureteronephrosis secondary to a left vesicoureteric junction stricture, complicated by multiple episodes of urinary tract infection.
Clinical examination revealed a mildly tender left-sided abdominal mass with no other localising signs, peritonism or cardiorespiratory compromise. Serological markers showed signs of acute kidney injury (creatinine 148 μmol/L from a baseline of 129 μmol/L) and raised inflammatory markers (C-reactive protein 54 mg/dL and procalcitonin 0.44 ng/mL). Preliminary urinalysis confirmed gross haematuria with pyuria (red blood cell count 149 cells/μL and white blood cell count > 2,000 cells/μL).
Initial radiographs did not reveal any definitive source of infection. Unenhanced computed tomography (CT) of the abdomen and pelvis (
Fig. 1
Unenhanced (a) axial and (b) reconstructed coronal CT images of the abdomen and pelvis. (c) US image of the left kidney.
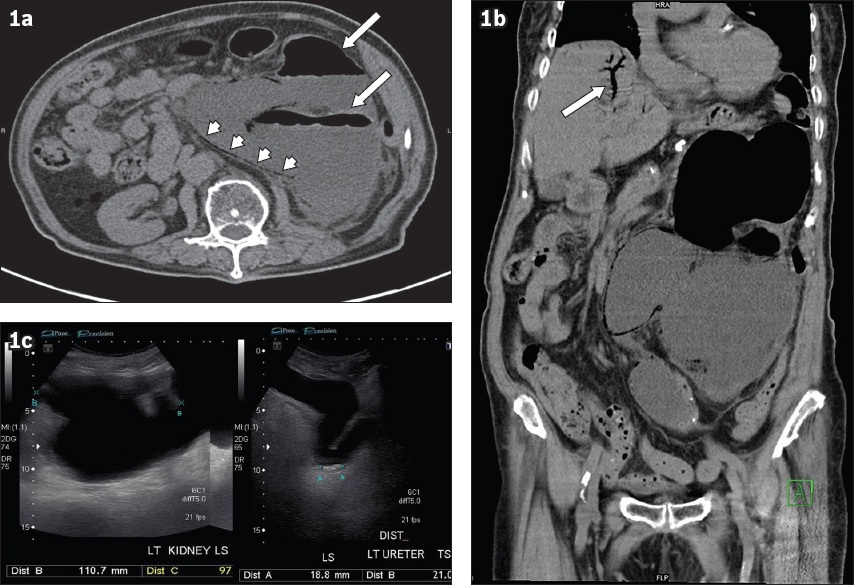
IMAGE INTERPRETATION
The unenhanced axial CT image of the abdomen and pelvis (
The unenhanced coronal CT image of the abdomen and pelvis (
Ultrasonographic evaluation of the kidney (
DIAGNOSIS
Severe left emphysematous pyelonephritis complicated by hepatic portal venous gas.
CLINICAL COURSE
The patient was admitted to an acute medical ward and successfully treated with intravenous antibiotics (initially piperacillin/tazobactam and amikacin, which was subsequently de-escalated to ampicillin). Urinary decompression of the infected obstructed system was offered from the outset but declined by the patient. Urine cultures on admission grew Klebsiella pneumoniae, and culture clearance was achieved prior to the patient’s discharge two weeks later.
DISCUSSION
The presence of portal venous gas is commonly thought to be a radiological indicator of abdominal catastrophe and a harbinger of poor outcomes, especially when associated with intestinal ischaemia.(1,2) Portal venous gas is most commonly attributed to gastrointestinal tract diseases, approximately 60% due to bowel ischaemia (
Fig. 2
Bowel ischaemia. An 84-year-old woman presented with abdominal distension. (a) Contrast-enhanced axial abdominal CT image shows diffuse portal venous gas in the liver with air-fluid levels within the portal vein (black arrow) and intrahepatic branches (black arrowhead). (b) CT image shows diffusely reduced small and large bowel wall enhancement with pneumatosis with multiple air-fluid levels in the bowel loops. No features of bowel perforation, free fluid or drainable abscess collection are identified. CT image shows critical stenosis of (c) the proximal coeliac artery (white arrow) and (d) superior mesenteric artery (white arrow). The imaging features are diagnostic of extensive bowel ischaemia involving the ascending and descending colon with associated portal venous gas secondary to critical arterial vascular compromise.
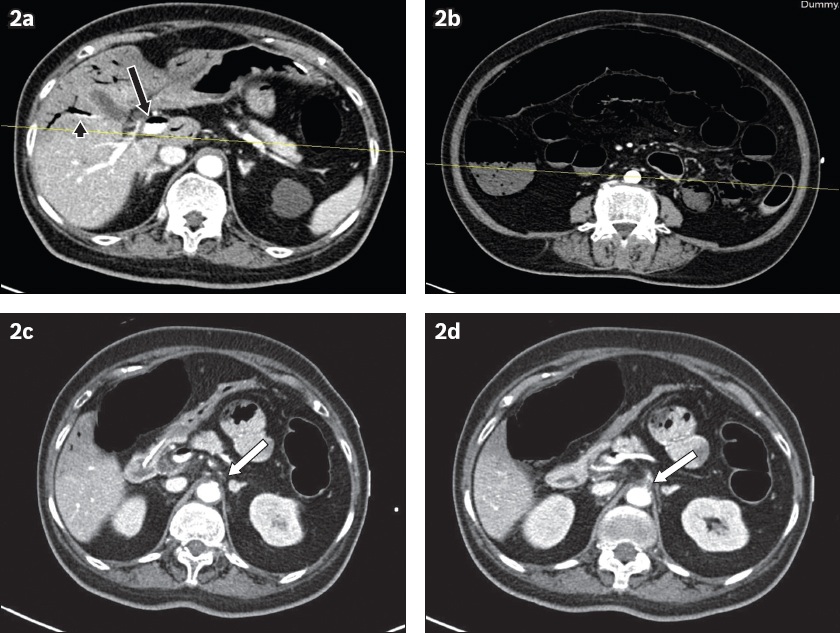
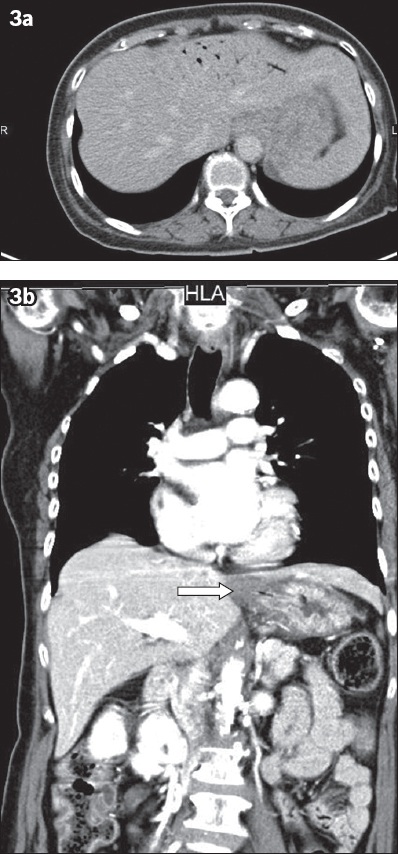
Fig. 3
Acute erosive gastritis. A 69-year-old woman presented with ingestion of a caustic agent. (a) Contrast-enhanced axial CT image of the upper abdomen following admission to the accident and emergency department shows diffuse gastric inflammation and small-volume portal venous gas involving the distal branches of the portal vein in the left hepatic lobe. No evidence of pneumoperitoneum or free peritoneal fluid is seen to suggest hollow viscus perforation. (b) Contrast-enhanced coronal CT image shows gastric inflammation, especially at the gastroesophageal junction (arrow). Gastroendoscopy confirmed the presence of severe and extensive ulcerations throughout the gastric mucosa. Ingestion of caustic fluids can lead to portal venous gas via a combination of gas production and compromise of the gastric mucosa, with the gas potentially dissecting and entering into the venous system.
To the best of our knowledge, there have only been four published cases of portal venous gas secondary to emphysematous pyelonephritis.(6-9) Given the absence of primary bowel involvement, the pathophysiology of gas in the hepatic venous system remains unclear.(6) Other seemingly unrelated pathologies such as chronic obstructive pulmonary disease and diabetes mellitus can also result in portal venous gas, although the exact mechanism remains unknown.
While portal venous gas, when associated with intestinal ischaemia, is a harbinger of poor outcomes,(1,2) reported cases of portal venous gas-related emphysematous pyelonephritis have had better outcomes, similar to our presented case, showing clinical improvement after medical treatment without the need for surgical intervention.(7,9)
It is important to distinguish portal venous gas from pneumobilia, a separate entity that has similar imaging appearances (described later in this article), as pneumobilia has a separate host of aetiological factors ranging from pathological processes such as cholangitis to iatrogenic, benign conditions such as post-sphincterotomy endoscopic retrograde cholangiopancreatography.
As part of the initial imaging workup, abdominal radiographs may reveal linear, branching lucencies overlying the hepatic parenchyma. However, the sensitivity of abdominal radiography in detecting portal venous gas is lower than that of CT. Additionally, portal venous gas may be less appreciated than pneumobilia, which typically has a greater volume of gas, rendering it more readily perceptible.
Ultrasonography (including Doppler flow imaging) has been proposed as an effective modality in the diagnosis and follow-up of portal venous gas.(10,11) Ultrasonographic features include direct visualisation of mobile intraluminal hyperechogenic foci in the portal vein, with marked shadowing or reverberation and sharp bidirectional spikes superimposed on the normal portal vein flow waveform. Previous attempts to pinpoint prognostic, predictive findings on ultrasonography have yielded mixed results.(2,12) Furthermore, limitations such as operator technique and patient habitus significantly impact diagnostic efficacy.
Cross-sectional imaging with CT is considered the most sensitive and informative modality in identifying portal venous gas and exploring potential aetiologies such as pneumatosis intestinalis in bowel ischaemia or generalised bowel dilatation in mechanical bowel obstruction, the former with a reported specificity of > 95%.(13,14) The classical appearance of portal venous gas on cross-sectional imaging involves linear, small-calibre, branching hypodensities of gaseous density in the portal vein and its tributaries, extending to within 2 cm of the liver capsule (
Fig. 4
Pancreatitis. A 76-year-old woman presented with epigastric pain and vomiting. (a & b) Unenhanced axial CT images of the abdomen demonstrating branching linear gas in the liver parenchyma and tributaries of the portal vein, extending to the liver edge. (b) CT image shows diffuse pancreatic parenchymal enlargement with oedematous changes (white arrow), poorly defined pancreatic borders and marked retroperitoneal fat stranding (white arrowheads), classic signs of acute pancreatitis. Multiple calcified gallstones (black arrow) can be seen within the marginally thick-walled gallbladder.
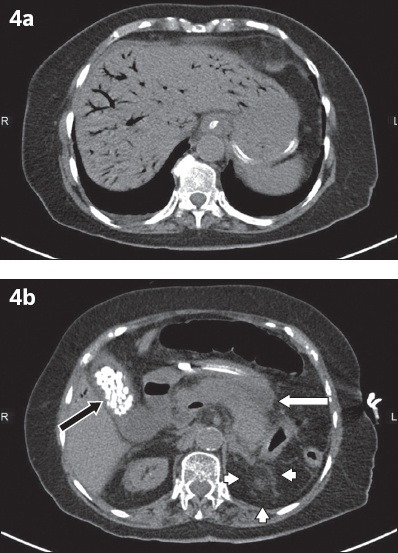
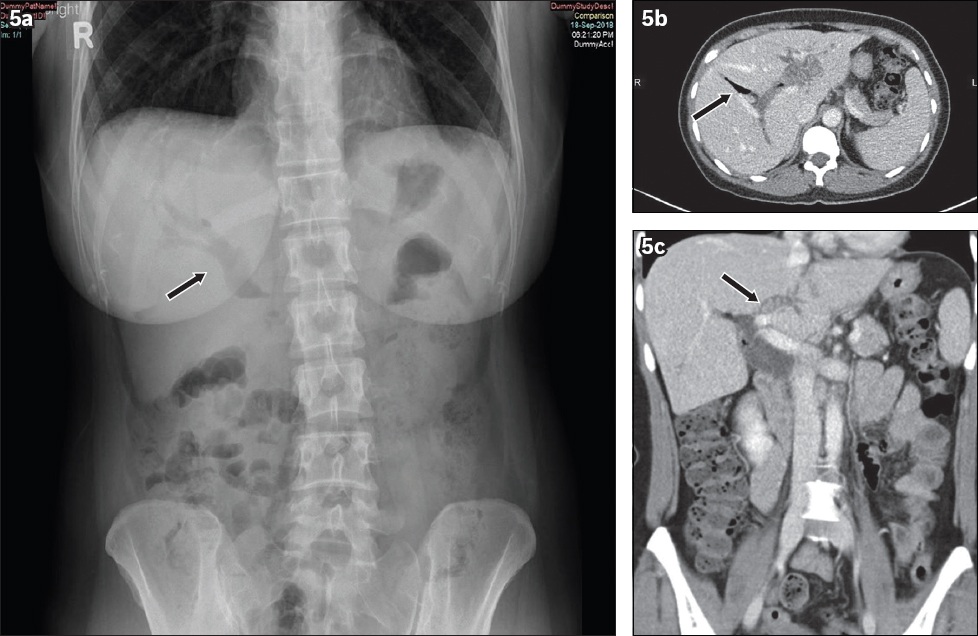
Fig. 5
Pneumobilia. A 42-year-old woman presented with right hypochondrium pain. (a) Erect abdominal radiograph shows branching linear lucencies projected over the intrahepatic and common bile ducts (black arrow). (b) Contrast-enhanced axial CT image of the abdomen demonstrates severe intra- and extrahepatic biliary dilation with centrally located gas (black arrow) in contrast to the typical, peripheral location of portal venous gas. (c) Contrast-enhanced coronal CT image shows several small iso-attenuating calculi within the left main intrahepatic biliary duct (black arrow). No other obstructing lesion within the common bile duct is noted.
In conclusion, portal venous gas should always raise concerns for a potentially life-threatening intra-abdominal pathology. Portal venous gas is not a disease entity but rather a consequence of intra-abdominal organs drained by one of the portal vein’s tributaries. Initial differentiation from pneumobilia, coupled with evaluation of the constellation of clinical and radiological findings, is paramount in order to arrive at the correct diagnosis and appropriately direct management.
Supplementary Material
SMJ-63-249.pdf


