Dear Sir,
The role of real-time polymerase chain reaction (RT-PCR) is pivotal in the diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The COVID-19 pandemic started from Wuhan, China, in December 2019, and the disease is still evolving.(1-3) False-negative RT-PCR results have been reported in human immunodeficiency virus (HIV) patients with COVID-19.(4) However, data on the features of COVID-19 in these patients is scarce.(5) We present a case of co-infection of HIV-1, herpes simplex virus-2 (HSV-2) and SARS-CoV-2 with false-negative RT-PCR in a critically ill patient.
The first case of COVID-19 in our country, Saudi Arabia, was confirmed on 2 March 2020, and more than 316,000 people had been infected and more than 3,900 patients had died at the time of writing.(6,7) In May 2020, at the peak of the COVID-19 outbreak in Saudi Arabia, a previously healthy 40-year-old woman was admitted to the emergency department with four days of fever (38.5oC), persistent cough, myalgia and dyspnoea. Her peripheral oxygen saturation was 70% on room air, and she had haemodynamic instability. Her score on the Glasgow Coma Scale was 9 out of 15, with no other neurological pathology. She was intubated, mechanically ventilated and transferred to the intensive care unit (ICU) designated for COVID-19. Laboratory findings were normal, apart from lymphocytopenia (0.69 × 109/L; normal range [NR] 1.1–3.2 × 109/L), and increased levels of C-reactive protein (706 mg/L; NR 0–5 mg/L) and lactate dehydrogenase (990 units/L; NR 100–190 units/L). The rest of the biochemical report was within normal limits.
The patient underwent a full diagnostic work-up for viral, bacterial and systemic disorders. Cardiac enzymes, electrocardiogram and echocardiography results were normal. Chest computed tomography (CT) depicted bilateral ground-glass opacities and consolidations (
Fig. 1
(a) CT image of the chest shows bilateral diffuse peripheral ground-glass opacities and consolidations with mainly lower lobe distribution. (b) Brain CT and (c) CT angiography images show diffuse white matter hypodensities in the frontal-parietal region, extending to the corpus callosum, basal ganglia and brain stem (mainly on the left side).
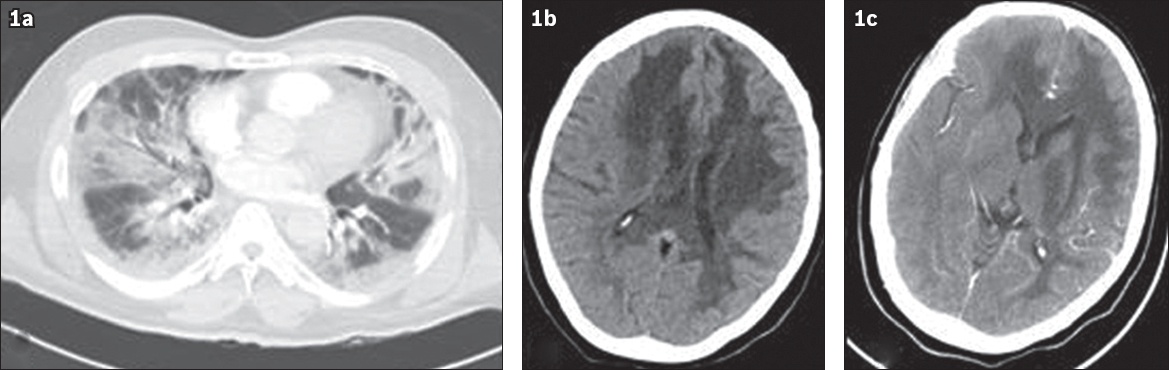
Emergency central nervous system (CNS) CT and angiography revealed diffuse white matter hypodensities and brain oedema (
On Day 3, the patient developed septic shock and multisystem organ failure. Antibiotics were upgraded to meropenem (2 g intravenously every eight hours) and vancomycin (1 g intravenously every 12 hours). Vasopressors and fluid resuscitation were initiated. The patient developed acute kidney injury with associated acidosis and hyperkalaemia, and underwent continuous renal replacement therapy as per Kidney Disease Improving Global Outcomes 2019 guidelines.(12) On Day 4 after ICU admission, the third RT-PCR test performed for COVID-19 was positive (31 copies for the E gene and 29 copies for the RdRp gene assays per reaction). Rescue therapies were considered; however, the patient developed ventricular tachycardia and cardiac arrest. No electrolyte disturbances were observed. Despite strenuous resuscitation efforts, the patient succumbed to the disease. Blood cultures for common bacterial and fungal infections were negative. CSF Cryptococcal and blood Toxoplasma gondii antigen serology tests were also negative. Cultures derived from endotracheal tube aspirates were negative for common bacteria, and microscopy examination, which was used for detection of Pneumocystis species, was also negative. In addition, tests performed for systemic disorders (i.e. autoimmune disorders, including antiphospholipid antibodies) were negative.
This brief case report, albeit with limitations, carries important messages. Life-threatening COVID-19 can present with acute respiratory distress syndrome, sepsis, multisystem organ failure, neurological manifestations and thromboembolic phenomena.(1-3) Notably, devastating CNS pathology, ranging from meningoencephalitis to stroke and acute disseminated encephalomyelitis, has been reported in patients with severe COVID-19.(13-15) In our patient, contrast brain CT did not depict any ring enhancement; however, the possibility of other diagnoses, such as toxoplasmosis or CNS lymphoma related to HIV, cannot be excluded. Unfortunately, magnetic resonance imaging was not performed as the patient deteriorated rapidly and could not be transported safely outside of the ICU. Also, the possibility of stroke remains a differential diagnosis and the detection of HSV-2 in the CSF cannot be ignored. This is a rare (< 2%) cause of HSV encephalitis and has a bad prognosis, although it is rare for HSV-2 encephalitis to present with brain oedema and mass effect.(16) We are uncertain how our patient was infected with SARS-CoV-2 and HIV, as her past medical history and list of conducts were unavailable. Hence, the exposure risk could not be properly evaluated, which is a major limitation of this case report, preventing its generalisability. Nevertheless, our patient had a relatively high HIV viral load with preserved CD4 count and CD4/CD8 ratio. Therefore, we speculate that our patient could have had acute HIV infection or HIV seroconversion illness, which is associated with CNS pathology,(17) although its link to COVID-19 is not yet elucidated.
In our case, cultures for fungal infections, microscopy for detection of Pneumocystis species, as well as serology antigen tests for Cryptococcus and Toxoplasma species were all negative; however, opportunistic infections cannot be definitively excluded. Initial empiric treatment could have included trimethoprim/sulfamethoxazole, but the decision was not made by the treating team. Despite all applied empiric and supportive therapies, the patient had a fulminant clinical course and succumbed owing to the development of cardiac arrhythmias, which led to cardiac arrest, although no electrolyte disturbances were observed. Hence, the possibility of COVID-19-related myocarditis or stress cardiomyopathy should be considered, as reported in other studies.(18-24) Postmortem autopsy reports were not available in our case, as it is not allowed by the current legislation in Saudi Arabia. In our untreated HIV patient, the false-negative result of RT-PCR for COVID-19 cannot be clearly attributed to the suppressive effect of anti-HIV-1 agents.(4,5) Stability issues and test fluctuations owing to insufficient viral material of the obtained nasopharyngeal specimen might have been responsible for the persistently negative RT-PCR results.(25) Notably, testing for SARS-CoV-2 could also be directed towards lower respiratory tract specimens (i.e. endotracheal aspirate and bronchoalveolar lavage specimens), although this remains controversial, or alternate specimens (i.e. stool), which may be helpful diagnostic adjuncts along with serology examinations.(26-28)
In our patient, the full picture of epidemiologic and exposure risk background was unavailable, which further complicated the evaluation of her immune system response to the viral infections. Scarce data exists in the literature on the laboratory and clinical features of COVID-19 in patients with active HIV infection.(4,5) The role of RT-PCR is pivotal in the diagnosis of SARS-CoV-2 infection, but in the face of inconclusive results, the integration of chest CT in our diagnostic arsenal remains mandatory.(29,30) Moreover, the natural course of SARS-CoV-2 viraemia remains obscure, as re-infections and/or recurrently positive RT-PCR results have been reported.(30-32)
In conclusion, co-infection of SARS-CoV-2 with other viruses may result in unpredictable dysregulations of the immune system response that are not fully understood. The diagnostic and therapeutic challenges are obvious. Further research is required to investigate co-infections of SARS-CoV-2 with other viruses, as well as the diagnostic performance of RT-PCR in such cases.
ACKNOWLEDGEMENTS
This study was approved by the Institutional Review Board of King Saud Medical City, Riyadh, Kingdom of Saudi Arabia (H-01-R-053, IORG0010374#, H1RI-07 May-20-01). Written informed consent was obtained from the patient’s legal representative postmortem for the publication of this study for scientific purposes.


