Abstract
The acutely obstructed airway is a medical emergency that can potentially result in serious morbidity and mortality. Apart from the latest advancements in anaesthetic techniques, equipment and drugs, publications relevant to our topic, including the United Kingdom’s 4th National Audit Project on major airway complications in 2011 and the updated American Society of Anesthesiologists’ difficult airway algorithm of 2013, have recently been published. The former contained many reports of adverse events associated with the management of acute airway obstruction. By analysing the data and concepts from these two publications, this review article provides an update on management techniques for the acutely obstructed airway. We discuss the principles and factors relevant to the decision-making process in formulating a logical management plan.
INTRODUCTION
Management of acute airway obstruction (AAO) in the adult surgical patient was a matter of concern highlighted in the 1998 United Kingdom (UK) National Confidential Enquiry into Perioperative Deaths and a subsequent journal editorial by Mason et al.(1,2) In 2011, the 4th National Audit Project (NAP4) in the UK, which was investigating anaesthesia-related major airway complications, included 50 obstructed airway cases.(3) Many of these patients were not ideally managed and some required an emergency surgical airway procedure and/or suffered serious sequelae, including brain damage and death. The American Society of Anesthesiologists’ (ASA) difficult airway algorithm, updated in 2013,(4) is also discussed. This article analyses and contributes to the data and concepts from NAP4 and the ASA algorithm, showing how the basic template from Mason et al(2) is still pertinent two decades on.
METHODS
The article focuses on causes of AAO in the adult surgical patient relating to an underlying pathology. Major airway complications occur for many reasons, including poor airway assessment, failure to create a strategy and failure to plan for failure.(3) Some of these reasons are considered preventable.
The Difficult Airway Society’s algorithm for unanticipated difficult airway specifically excludes patients with AAO of the upper airway.(5) This is partly due to the fact that most cases of AAO are anticipated and require complex airway management strategies. The ASA’s difficult airway algorithm provides guidelines for both anticipated and unanticipated cases, but lacks specific guidelines on the management of AAO.(4) Therefore, this article analysed the generic basic management choices of these guidelines, and extrapolated the choices and reasoning to be applied to AAO use. Logical Plans A, B and C were, in turn, formulated based on Mason et al’s(2) algorithm for AAO. A review of the literature found that it supported the principles and applicability of both these algorithms. Deviations from them may lead to serious morbidity and mortality, as highlighted in the recent NAP4 report.(3) Recognition and management of AAO requires a multidisciplinary approach, as there are many surgical causes of AAO, and may involve emergency department personnel, and ear, nose and throat (ENT), and thoracic surgeons. Usually, input from the anaesthesiologist is required to provide general anaesthesia for surgery or as part of the resuscitation team in cases of imminent complete AAO. On occasion, an emergency surgical airway procedure performed under local anaesthesia by the ENT surgeon is warranted.
In airway management, it is vitally important to have a Plan A, B and C, i.e. two backup plans if the initial Plan A fails. We routinely use the template proposed by Mason et al,(2) as it satisfies and incorporates two sets of preconditions essential for safe management of AAO: firstly, establishing the cause, location and degree of the obstruction; and secondly, determining which elements of the four ASA basic management choices are most appropriate to secure the airway.(4) It is vital to understand how each of these choices will affect the presenting AAO case, possibly in a deleterious way.
We take the opportunity to update the recommendations proposed by Mason et al(2) in 1999. Firstly, we place less emphasis on gas induction following reports of morbidity using that technique,(3) although we acknowledge that it is still an important option in AAO management. Secondly, there is a recent recognition that the use of muscle relaxants may be beneficial in certain cases of difficult airway and airway obstruction.(6,7) This is further supported by the introduction of sugammadex in 2008.(8) Thirdly, we discuss the recent use of videolaryngoscopes. Lastly, we discuss in greater detail the place of extracorporeal oxygenation in the management of AAO.
CAUSES, LOCATION AND DEGREE OF OBSTRUCTION
There are many surgical causes of AAO in the literature. Infective causes include Ludwig’s angina,(9) tonsillar or pharyngeal wall abscess,(10) and acute epiglottitis.(11) Inflammatory causes include subglottic/tracheal stenosis,(7) goitre(12) and lingual tonsillar hypertrophy.(13) Neoplastic causes include laryngeal or tracheobronchial cancers,(14) and those associated with mediastinal mass syndrome.(15) 80% of emergency awake tracheostomies are related to malignant disease.(16) Laryngospasm, which occurs in less than 1% of patients undergoing general anaesthesia,(17) is not included in this article, as it is usually an unanticipated phenomenon due to an exaggerated protective airway reflex.
Most cases of AAO are anticipated from symptoms, signs and results of medical investigations. Symptoms include shortness of breath, hoarseness of voice, noisy breathing and postural symptoms. The latter include sleeping on multiple pillows, preferring to lie on one side or waking up at night feeling breathless. Severity of symptoms is also affected by the speed of onset. Signs indicating difficult intubation may be due to the underlying disease process. Stridor or ‘hot potato voice’, which indicates supraglottic involvement or tumour, is traditionally associated with a reduction of the tracheal diameter of more than 50%. Bedside tests may indicate difficult face mask ventilation or intubation. Independent predictors for difficult face mask ventilation are: obesity, aged 55 years and above, history of snoring, lack of teeth, the presence of a beard, Mallampati Class III or IV, and abnormal mandibular protrusion test.(18) Predictors for difficult intubation include: decreased interdental and thyromental distances, increased Mallampati scores, and decreased neck movement.(19,20) One study demonstrated 12 independent predictors of combined difficult face mask ventilation and laryngoscopy: aged 46 years and above; body mass index ≥ 30; male gender; Mallampati Class III or IV; neck mass or radiation changes; limited thyromental distance; sleep apnoea; limited cervical spine mobility; limited jaw protrusion; thick neck; presence of teeth; and beard.(21) Patients with lingual tonsillar hypertrophy may not present with symptoms and signs, as their tonsillar tissue is slow-growing and hidden behind the base of the tongue. These patients may, therefore, present as unanticipated cases of difficult airway, which may lead to serious morbidity and even death.(13,22)
Diagnostic information is important to determine the location and degree of AAO, and whether it is an annular and/or fixed, or a large pedunculated or ‘ball valve’ type. The latter requires the patient to maintain spontaneous ventilation (SV). These can all be investigated by flexible nasendoscopy and radiological imaging. Flexible nasendoscopy is a noninvasive test and does not require airway topicalisation, thereby avoiding airway irritation that can potentially cause laryngospasm and loss of the airway.(23-28) It provides a bird’s eye view of the supraglottis and allows diagnostic information to be obtained, including the accessibility of the lesion and degree of obstruction. NAP4 reported that flexible nasendoscopy was “not used to evaluate the airway as often as it was indicated”.(3) Radiography, computed tomography and magnetic resonance imaging of the airway are helpful, but may not be practical or appropriate in the presence of severe AAO.
The location of the lesion is important as it determines if the anaesthesiologist can access and bypass it with a given airway device or technique. The various locations can be broadly divided into oropharyngeal, laryngeal and supraglottic structures, and upper and lower tracheal regions. Obstructive lesions in the oropharynx may be bypassed from above with nasal fibreoptic intubation or from below with cricothyroidotomy or surgical tracheostomy. Large obstructive lesions in the larynx and trachea may prohibit the use of certain airway techniques. Awake fibreoptic intubation (AFOI) may cause a ‘cork in a bottle’ obstruction or laryngospasm in cases of severe AAO.(23-28) A tracheostomy may not be able to access and bypass large or low-lying lesions (e.g. retrosternal goitres or tracheobronchial lesions).
A relatively common upper tracheal lesion is the goitre. These are usually chronic lesions but, in rare circumstances (< 1%),(29) they can present as AAO with considerable management difficulties for the anaesthesiologist, and ENT and thoracic surgeons.(12,30) A goitre can cause a mass effect on surrounding structures, resulting in Pemberton’s sign when the gland acts as a ‘thyroid cork’ at the thoracic inlet and AAO.(31) If large, it can present clinicians with various challenges. Firstly, laryngoscopy may be difficult.(32) Secondly, the trachea may be compressed and deviated. Thirdly, the goitre may be an obstacle to a surgical airway procedure. Lastly, the goitre may cause a mediastinal mass syndrome. If a surgical airway procedure is needed in patients with large goitre presenting with AAO, the procedure may be difficult or impossible, although there have been successful cases.(33,34) In cases where a surgical airway procedure would be unable to bypass the obstruction, pre-induction cardiopulmonary bypass may be required.
BASIC MANAGEMENT CHOICES
The 2013 ASA algorithm requires the anaesthesiologist to consider the relative merits and feasibility of the four basic management choices, i.e. airway devices and techniques used to secure the airway.(4) These are: (a) awake or asleep intubation; (b) SV or ablation of SV (e.g. applying positive pressure ventilation to paralysed patients); (c) non-invasive or invasive airway devices; and (d) direct or indirect laryngoscopy to facilitate tracheal intubation.(4) The decision-making process in formulating logical (and safe) Plans A, B and C are presented later in this article.
Awake or asleep intubation
The decision to keep a patient awake or asleep is derived from predictions of the ability to maintain oxygenation/ventilation and secure the airway. Difficult face mask ventilation requires the use of an oral airway or two hands;(35) the incidence of difficult and impossible face mask ventilation is 1.4% and 0.15%, respectively.(36) Difficult intubation is commonly defined as a Cormack-Lehane view of Grade 3 or 4 on direct laryngoscopy,(37) and difficult and failed intubation are similarly rare (5.8% and 0.1%, respectively).(20,38) Bedside airway assessments used to predict these difficulties have low to moderate predictive power.(20) In general, the majority of patients are easy to ventilate and intubate, and most patients with a normal appearance can be intubated after induction of anaesthesia (asleep intubation).
Anaesthesia causes airway obstruction due to a loss of muscle tone, suppression of protective arousal responses(39) and decrease in respiratory reserve (due to atelectasis, right-to-left shunt, decrease in functional respiratory capacity, alveolar collapse and airway closure).(40-42) Therefore, as the ‘margin of safety’ is narrowed, full pre-oxygenation with the ‘three-minute tidal volume’ or ‘eight deep breath’ technique and transnasal oxygen insufflation is beneficial, as it prolongs safe apnoea time.(43,44) Even if a difficult airway is encountered, 90% of patients with Cormack-Lehane Grade 3 views (more common than Grade 4 views) can be successfully intubated with the aid of standard airway adjuncts such as the bougie and/or McCoy laryngoscope.(45) However, the incidence of difficult airway in ENT is higher in surgical patients than the general population (3.5%–15.7% and 2.5%, respectively).(46) This is especially so if the surgery is for cancer.
If face mask ventilation or intubation is predicted to be difficult, and attempts are at high risk of morbidity, the airway should be secured before induction of anaesthesia (awake intubation). This maximises the ‘margin of safety’, as airway muscle tone and reflexes are maintained,(47) and respiratory function is unaffected by anaesthetic agents. It also avoids the risk of a post-induction ‘cannot intubate, cannot ventilate’ (CICV) scenario, which has an incidence of 0.01%–0.17%.(3,48) The two most common techniques are AFOI and awake tracheostomy, although they are not without risks.(3) Rarely are other techniques used in awake patients with AAO, such as direct or indirect laryngoscopy,(49,50) rigid bronchoscopy(51) and femoral vessel cannulation for cardiopulmonary bypass.(15,52)
Preserved or ablated spontaneous ventilation
SV is preserved in the awake state or during anaesthesia after careful titration of intravenous or volatile agents. The latter, in theory, allows a ‘bailout’ exit strategy in the event of failure to secure the airway or total airway obstruction, by allowing the patient to wake up with cessation of delivery of the volatile agent. Gas induction with sevoflurane is performed in a slow, stepwise manner or by starting at a high 8% concentration. However, it should be noted that the minimum alveolar concentration of sevoflurane for endotracheal intubation is much higher than for surgical incision (4.5% vs. 1.7%–2.1%, respectively).(53-55)
Under acceptable intubating conditions, induction takes approximately six minutes using 7% sevoflurane in normal patients(56) but much longer in patients with AAO, as the obstruction prevents delivery of the volatile agent. Furthermore, induction and intubation with 8% sevoflurane without the use of muscle relaxants is associated with a higher failed intubation rate(57) and up to 10%–25% of upper airway complications, such as breath-holding and coughing.(58,59) In patients with AAO, laryngospasm is more common.(17) NAP4 also reported several cases of failed gas induction leading to serious morbidity, including airway obstruction, laryngospasm, failed intubation necessitating surgical airway procedure, and cardiac arrest.(3) It cautioned that gas induction may fail with loss of airway and failure to wake up, and recommended a clear and ready rescue plan.
Waking a patient up, and ensuring that he can maintain adequate airway and respiratory function thereafter, is not always possible for several reasons. The patient may have poor respiratory reserve due to pre-existing comorbidities and an inability to comply with full pre-oxygenation, both of which quicken the onset of critical haemoglobin desaturation.(60) The return of airway muscle tone and SV is also suppressed by residual anaesthetic drugs. In simulations of complete airway obstruction following gas induction, there are conflicting results as to which volatile agent offers the fastest wake-up time. One study showed that sevoflurane (with its lower solubility and slower redistribution) was found to decline three times slower than halothane, resulting in slower wake-up time.(61) Another study showed faster wake-up time with sevoflurane.(62) If SV is ablated by a sufficiently large dose of intravenous anaesthetic agent and/or the use of muscle relaxants, the ability to wake the patient may also be lost.
The main advantages to using muscle relaxants are that they may improve face mask ventilation and intubating conditions.(63-66) Further points for consideration are:
-
Patients with fixed, annular laryngotracheal stenosis. One study showed that muscle paralysis improved ventilatory dynamics compared with SV.(7) This should be distinguished from cases of large, pedunculated airway lesions, where muscle paralysis and positive pressure ventilation must be avoided, as they may cause a ‘ball-valve’ type obstruction.
-
Choice of muscle relaxant. Traditionally, suxamethonium was used for patients with difficult airway due to its fast onset and offset of action. If failed intubation occurred, the patient would regain airway muscle tone and resume SV. However, Benumof et al reviewed clinical mean duration times of suxamethonium and related it to calculated apnoeic times following sudden complete airway obstruction.(60) Calculations were based on a model of a healthy 70 kg adult under anaesthesia, after full pre-oxygenation. Times to reach critical haemoglobin desaturation (SaO2 < 80%) and functional recovery of suxamethonium (mean time for single twitch height to recover to 50%) were similar, i.e. 8.7 minutes and 8.5 minutes, respectively. However, in patients with AAO who are older and often have comorbidities, critical desaturation would occur much earlier, exposing the patient to unacceptable levels of hypoxia. One study even showed a mean duration time of 13.3 minutes for suxamethonium.(67) Historically, longer-acting muscle relaxants are not used due to the inability to immediately reverse deep block if bailout is required. A new strategy is to use a rapid-sequence dose of the intermediate-acting rocuronium (1.2 mg/kg) and follow this with its reversal agent, sugammadex (16 mg/kg), if bailout is required. This combination gives a similar onset but faster offset time compared with suxamethonium.(68,69) Sugammadex has been used to ‘rescue’ intense neuromuscular block after induction of patients with AAO;(70,71) however, these cases were criticised for an overreliance on the drug’s ability to guarantee rapid reversal, and a lack of logical induction, intubation and exit strategies.(72-79) Other considerations include the cost and the time to find and draw-up sugammadex,(80) and the potential of developing negative pressure pulmonary oedema if the AAO remains unresolved but breathing resumes, generating a large, negative intrathoracic pressure.
-
NAP4 recommends the use of muscle relaxants in CICV cases as they may resolve failure to ventilate caused by laryngospasm and aid mask ventilation.(3)
In symptomatic patients with large mediastinal masses or tracheobronchial lesions, maintaining SV and avoiding the use of muscle relaxants is considered the safest option. The resulting negative intrathoracic pressure helps to keep the intrathoracic airways open.(15,52) Tracheal intubation can be achieved either by awake (e.g. AFOI)(81,82) or asleep intubation, gas induction or carefully titrated target-controlled propofol infusion.
Noninvasive or invasive airway devices
In the UK, most patients undergoing general anaesthesia have their airways managed with noninvasive devices, such as a laryngeal mask airway (56%) or tracheal tube (38%).(3) Supraglottic airway devices have been used successfully for fixed annular obstructive lesions such as subglottic and tracheal stenosis.(7,83,84) However, in head and neck surgery, due to the shared airway, a tracheal tube is usually indicated.
In upper airway obstruction, if flexible nasendoscopy and radiological imaging show that the tracheal tube cannot access and bypass the lesion, another airway device or technique is indicated. In AAO involving the larynx, this usually indicates a procedure involving the insertion of an invasive airway that will bypass the obstruction from below, such as cricothyroidotomy or tracheostomy. Both procedures can be performed electively (prophylactically) in the anticipated difficult airway, or in the emergency setting. NAP4 contained 58 attempted emergency surgical airway procedures: 9 (16%) failed and 4 (7%) patients died.(3) Cricothyroidotomy can be classified into small bore (≥ 2 mm), large bore (≥ 4 mm) or surgical (≥ 6 mm).(85) Small-bore cricothyroidotomy requires a high-pressure oxygen source like a jet ventilator using low or high frequency. In order to prevent barotrauma, the presence of a leak path (patent expiratory pathway) is essential. Large-bore and surgical cricothyroidotomy allow ventilation through the device itself, hence only require a low-pressure oxygen source. Leaks must not be present when these devices are used, so the cuff component of the device should be inflated in cases where the lesion has not caused total obstruction.
Despite the risk of barotrauma, subglottic high-frequency jet ventilation via small-bore cricothyroidotomy has been used electively for patients with severe airway compromise (6% of patients had impossible face mask ventilation).(86) Ross-Anderson et al(86) achieved adequate ventilation in 95% of their patients using this technique, with minor complications occurring in 20%. They used an automated jet ventilator with a pause pressure function, a pre-set airway pressure limit above which the alarms are set off and the ventilator stops jetting, thus minimising the risk of barotrauma. Jet ventilation must be used with extreme caution in patients with AAO due to the complex interplay between the various components of jet ventilation that affect the resultant minute volume and airway pressure. These components consist of: route (supraglottic, transglottic and translaryngeal/tracheal), ventilator parameters (driving pressure, frequency and inspiratory time), ventilator modalities (low, high or superimposed high frequency) and the effects of the stenosis itself. The degree of stenosis affects the degree of gas trapping, gas exhaust and entrainment.(87-89) NAP4 reported a 65% failure rate with emergency needle cricothyroidotomy, but this is likely to be an overestimate as successful attempts would not have been reported.(3)
NAP4 emphasised that awake tracheostomy may offer a safer alternative to tracheal intubation after induction of anaesthesia, and that it should actively be considered.(3) In addition, there were CICV cases that lent themselves well to AFOI or awake tracheostomy. Indications for awake tracheostomy for AAO of the upper airway include: severe stridor, large tumour, fixed hemilarynx, gross anatomical distortion and larynx not visible on flexible nasendoscopy.(2) Emergency awake tracheostomy is lifesaving, but associated with a 7.8%–8.2% complication rate.(16,90) NAP4 reported 29 successful first-choice emergency tracheostomies,(3) with 11 being performed for true emergencies (i.e. patients in extremis). There were 2 (7%) deaths. One was due to the tracheostomy not being able to bypass a low-lying lesion and the other was from pre-procedure severe hypoxia. Other studies found a 0%–2% incidence of death following emergency tracheostomy.(16,90)
Direct or indirect laryngoscopy
Sir Robert Reynolds Macintosh introduced his laryngoscope blade in 1943 to elevate the epiglottis to facilitate intubation.(91) It has been the mainstay for anaesthesiologists ever since, with direct laryngoscopy relying on the alignment of the oral, pharyngeal and laryngeal axes to view the glottis.(92) The quest for newer techniques and technologies to overcome difficult laryngoscopy led to the development of indirect laryngoscopy, allowing the anaesthesiologist to ‘look around the corner’ to view the glottis. The prototype of the intubating fibreoptic bronchoscope (FOB) was described in 1967,(93) but only in 2001 was the first of a new generation of videolaryngoscopes (GlideScope®; Verathon Company, Bothell, WA, USA) introduced. The 2003 ASA difficult airway algorithm was then updated a decade later with the addition of a fourth basic management choice of whether to use direct laryngoscopy or indirect laryngoscopy.(4,94)
There are three types of indirect laryngoscopy devices: nonguided blades (similar to the Macintosh laryngoscope); guided blades; and optical stylets. The latter are either flexible, e.g. FOB, or rigid, e.g. Bonfils fibrescope (Rusch Inc, Duluth, GA, USA).(95,96) They allow the anaesthesiologist to ‘look around the corner’ by employing the use of fibreoptics, lenses, prisms, mirrors or a micro-camera. Theoretical advantages of indirect laryngoscopy over direct laryngoscopy are improved glottic views and increased chances of successful tracheal intubation, either as a primary or rescue technique after initial failure with direct laryngoscopy.
If asleep intubation is deemed safe, there are three functional characteristics that need to be considered before choosing the indirect laryngoscopy device in AAO cases:
-
Provision of an indirect view. All three types of devices are able to ‘look around the corner’ to view the glottis. Although nonguided blades may succeed in obtaining a full view of the glottis, correct placement of a tracheal tube may be difficult or impossible, even with the help of intubating aids such as stylets or bougies. 35% of failures to intubate with the GlideScope were associated with a Cormack-Lehane Grade 1 or 2 view.(97)
-
Ability to guide a tracheal tube to the indirectly viewed glottis. Both guided blades and optic stylets have this ability. The former has an inbuilt ‘conduit’, whilst the latter allows railroading of a preloaded tracheal tube along its slender shaft.
-
Ability to manoeuvre around an obstructing supraglottic lesion. Nonguided and guided blades are too bulky to allow much movement once inserted into the oral cavity; however, the thin and slender stylets can be manipulated in various directions to reveal a potential pathway to the glottis.
Two recent review articles summarised the performance of indirect laryngoscopy devices. One concluded that, in predicted or known difficult airways, the best-performing devices were the Bonfils, CTrach™ and GlideScope.(96) The other review recommended the Airtraq®, CTrach, GlideScope, Pentax AWS® and Video Macintosh for patients with predicted difficult airways.(98) The latter review recommended the Airtraq, Bonfils, Bullard™, CTrach, GlideScope and Pentax AWS for known difficult intubation (Cormack-Lehane Grades 3 and 4). Results are difficult to interpret due to the lack of homogenous data. Unsurprisingly, there are no comparative studies investigating indirect laryngoscopy in patients with AAO due to the ethical issues involved. One study assessing GlideScope performance revealed that failure was most strongly associated with factors found in AAO: altered neck anatomy with a surgical scar, radiation changes and mass.(97)
Intubation using nonguided and guided blades involves inserting the device into the oral cavity, then steering a hollow tracheal tube towards and through the glottis, keeping the latter patent. Intubation using a flexible optical stylet requires a different sequence of events. The FOB needs to be inserted into the oral cavity (directly or via the nose) and then towards and through the glottis, and into the trachea. At this point, it can obstruct the airway. The preloaded tracheal tube is then railroaded over the FOB and passed into the trachea. Flexible AFOI is suitable for bypassing oropharyngeal lesions in elective cases, specifically base-of-tongue lesions, as these are associated with CICV (e.g. large tumours and lingual tonsillar hypertrophy).(3) It is less suited for supraglottic or tracheal stenotic lesions, as the shaft of the FOB itself may exacerbate the pre-existing obstruction. Rigid stylets have the advantage of allowing one-handed operation, while the other hand can optimise the oropharyngeal airspace (e.g. using the thumb or laryngoscope). They can also be used to gently push an obstructive lesion out of the way.
In mild cases of obstruction, AFOI has been used successfully.(99-101) However, in severe AAO, AFOI should be avoided, due to a number of factors. AFOI requires a calm, cooperative patient, which is unlikely in severe AAO. Airway topicalisation or intravenous sedation may cause loss of airway muscle tone or laryngospasm.(23-26) Navigating the FOB may be technically challenging or impossible. If a friable tumour is the cause of the AAO, there is the risk of bleeding and a resultant acute loss of airway if contact is made with the FOB. Crucially, the passage of the FOB through a tight obstruction may be difficult or impossible, and risks causing a ‘cork-in-a-bottle’ scenario.(27,28) Lastly, railroading the tracheal tube may also prove difficult or impossible in severe AAO.
FORMULATING PLANS A, B AND C
After a failed initial attempt at tracheal intubation in an anaesthetised patient, the overriding priority is ventilation and oxygenation of that patient. However, international difficult airway algorithms differ greatly in their Plans A, B and C, and each has its limitations in managing AAO.(102) The UK Difficult Airway Society algorithm is based on low-strength evidence (expert committee reports or opinions, or clinical experience of respected authorities) with Strength D recommendations.(5,103) Similarly, the ASA algorithm consists of predominantly Category B evidence (observational studies or randomised controlled trials without pertinent comparison groups).(4) When Cook et al(30) invited nine international airway experts to give their opinion on the management of AAO due to a retrosternal goitre, there was little consensus on Plan A. However, their Plan Bs almost unanimously recommended a rigid bronchoscope to help secure the airway.
Regardless of which difficult airway algorithm is adopted, NAP4 highlighted several requisites for the safe management of AAO:(3) (a) the need for skill and cooperation between the anaesthesiologist and airway surgeon; (b) a fully equipped environment with full surgical, anaesthetic and nursing support; (c) an operating theatre as the ideal location; (d) active consideration of awake tracheostomy as a safer alternative in appropriate cases; and (e) an instantly available surgical airway procedure, if it is part of a backup plan.
Once Plans A, B and C have been formulated, it is important to avoid fixation errors that may result in a failure to recognise and abort a plan that is not working, and be able to move to another potential solution.(3) One high-fidelity simulation study showed that there were persistent deviations from the ASA difficult airway guidelines in the management of CICV, particularly in the need to progress to a surgical airway procedure.(104) They consisted of repeated intubation attempts, use of an FOB, bypassing the use of a laryngeal mask airway and failure to call for help early. These deviations occurred despite participants’ involvement in an initial CICV scenario, followed by an intense one-hour debriefing session focusing on the correct guidelines and with cricothyroidotomy teaching.
Our template for Plans A, B and C (
For patients with AAO in the upper airway (e.g. a laryngeal tumour or acute epiglottitis):
Fig. 1
Chart shows airway management strategy for acute airway obstruction using a template for Plans A, B and C. Movement may take place from Plan A to Plan C depending on patient and operator factors (broken arrow). AFOI: awake fibreoptic intubation; CICV: cannot intubate, cannot ventilate; CPB: cardiopulmonary bypass.
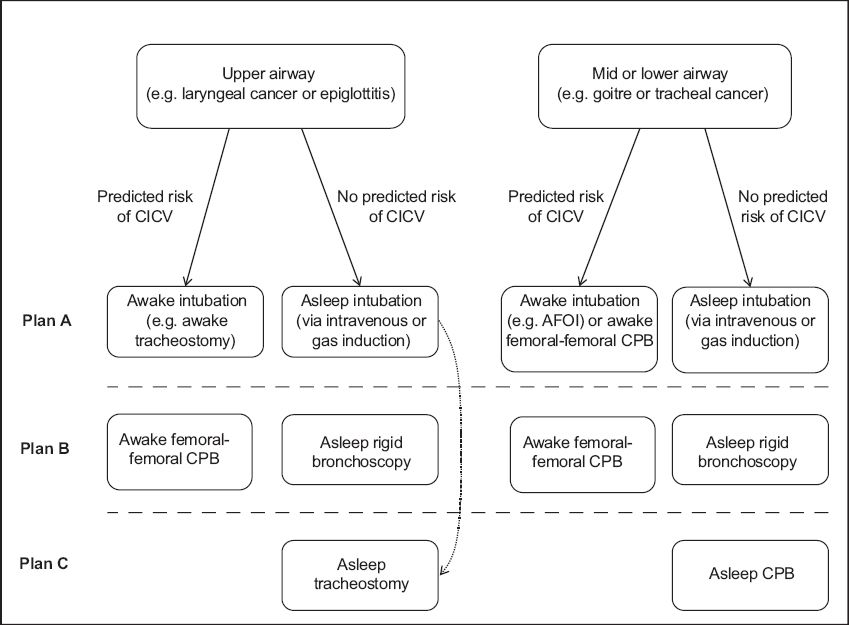
-
No predicted risk of CICV
-
Plan A: asleep intubation (intravenous or gas induction). After induction, direct or indirect laryngoscopy is performed by the anaesthesiologist, and tracheal intubation attempted.
-
Plan B: rigid bronchoscopy.(105) This is performed by the airway surgeon and helps secure the airway. It allows implementation of conventional or jet ventilation, tamponades airway lesions and allows surgical procedures, both diagnostic and therapeutic, to be performed. It also helps promote a controlled environment, as the airway is secured and ventilation is taking place prior to Plan C, if the latter is necessary.
-
Plan C: asleep tracheostomy. Occasionally, rigid bronchoscopy as Plan B is omitted and an emergency asleep tracheostomy is performed due to clinical necessity.
-
-
At risk of CICV
-
Plan A: awake intubation. Awake tracheostomy is most commonly performed,(16,90) as AFOI is often contraindicated (see section ’Direct or indirect laryngoscopy’).
-
Plan B: cardiopulmonary bypass.
For patients with AAO in the mid-trachea and lower airway (e.g. large retrosternal goitre and tracheal tumour):
-
-
No predicted risk of CICV
-
Plan A: asleep intubation (intravenous or gas induction). Some authors strongly recommend preservation of SV as the negative intrathoracic pressure will help relieve any intrathoracic obstruction. This is achieved by gas induction and avoidance of muscle relaxants.(15,52)
-
Plan B: rigid bronchoscopy.
-
Plan C: cardiopulmonary bypass.
-
-
At risk of CICV:
-
Plan A: awake intubation. This is most commonly AFOI, but there are dangers of a ’cork-in-a-bottle’ scenario or laryngospasm.(23-28) Awake tracheostomy is very difficult (33,34) or impossible due to the underlying pathology (e.g. large retrosternal goitre) or being unable to bypass a low-lying central airway obstructive lesion.
-
Plan B: cardiopulmonary bypass.
-
In rare cases of AAO in which it is not possible to access and bypass the lesion, failure to secure the airway may lead to a CICV scenario. Methods of maintaining oxygenation of the patient then rely on techniques that bypass the need for lung ventilation (e.g. cardiopulmonary bypass or extracorporeal membrane oxygenation). These blood oxygenators are connected to the patient via awake femoral vessel cannulation. Some researchers strongly recommend establishing cardiopulmonary bypass by cannulation under local anaesthesia and before induction of general anaesthesia.(15,52) This is due to the risk of sudden cardiorespiratory collapse and the time needed for the bypass machine to be fully functional. Due to the logistical difficulties in setting up cardiopulmonary bypass, some authors have only established it after securing the airway,(81,82) rather than before.(106-109) Several cases of its successful use in central airway obstruction (including mediastinal mass syndrome) or symptomatic patients with large retrosternal goitres have been reported.(15,81,82,106-109)
CONCLUSION
The NAP4 report highlighted the poor management of AAO and subsequent complications in these patients.(3) ASA’s basic management choices and the algorithm by Mason et al provide the anaesthesiologist with a suitable template for formulating a logical primary and backup airway strategy.(2,4) In addition, recent advances (such as the use of muscle relaxants and sugammadex, videolaryngoscopy, jet ventilation, and cardiopulmonary bypass) offer more options for anaesthesiologists. The decision-making process in AAO management should be based on individual circumstances, including patient status, location and extent of the obstruction, pathology, degree of urgency, and availability of expertise and facilities.


